
Life Science Module—Activity 1
Activity Summary
In this activity, students investigate the range of
conditions that selected animal and plant species need to
survive in an estuary. They examine data for abiotic
factors that affect life in estuaries—salinity, dissolved
oxygen, temperature, and pH. Students use archived da-
ta (trend analysis graphs) and real-time conditions at the
Elkhorn Slough National Estuarine Research
Reserve (NERR) to predict whether a particular animal
or plant species could survive in an estuary.
Learning Objectives
Students will be able to:
1. Describe three types of estuarine environments.
2. Describe the particular environmental conditions
necessary for organisms to survive in an estuary.
3. List four principal abiotic factors that influence the
survival of aquatic life in estuaries.
4. Determine the range of pH, temperature, salinity,
and dissolved oxygen tolerated by some common
estuarine species.
Grade Levels
9-12
Teaching Time
3 (55 minute) class sessions + homework
Organization of the Activity
This activity consists of 4 parts which help deepen
understanding of estuarine systems:
The Estuarine Environment
Surviving Changes: Abiotic Factors that Affect Life
Surviving in an Estuary: Extreme Conditions
Optional: Investigating Other NERRS sites
Featured NERRS Estuary:
Elkhorn Slough National Estuarine
Research Reserve, CA
http://nerrs.noaa.gov/Reserve.aspx?ResID=ELK
Teacher Guide—Life Science Module
Activity 1: Survival in an Estuary
Background
This activity introduces students to the
nature of estuaries, estuarine environmental
factors, and four important abiotic
factors—pH, temperature, dissolved
oxygen, and salinity—and how they vary in
estuaries. The study centers on Elkhorn
Slough National Estuarine Research

2
Life Science Module—Activity 1
When algae naturally begin to increase in estuaries as
they may do when days lengthen and the water
temperature rises in spring, pH levels tend to rise.
Respiration, on the other hand, releases CO
2
into the
water, thus resulting in a lower pH, so pH levels may
drop during the summer nights.
All aquatic organisms have a pH range to which they are
adapted. Outside of this range, critical biological
processes may be disrupted, leading to stress and death.
Most organisms cannot live below a pH of 5 or above a
pH of 9. Additionally, pH is used to monitor safe water
conditions. Once the background range of pH has been
established, a rise or fall in pH may indicate the release
of a chemical pollutant, or an increase in acid rain.
Additionally, pH affects the solubility, biological
availability, and toxicity of many substances. For
example, most metals are more soluble, and often more
toxic, at lower pH values.
Temperature
Just knowing the temperature of the water in an estuary
can give us a pretty good idea of how healthy it is. One
important thing we can tell from water temperature is
how much oxygen can be dissolved into the water.
Dissolved oxygen is critical for the survival of animals
and plants that live in the water. As the water
temperature increases, the amount of oxygen that can
dissolve in the water decreases. For example, 100 % sat-
urated fresh water at 0°C contains 14.6 mg of oxygen
per liter of water, but at 20°C, it can only hold 9.2 mg of
oxygen per liter. Because dissolved oxygen is critical for
survival, seasonal water temperature (and dissolved
oxygen) is an important indicator of habitat quality for
many estuarine species.
The temperature of the water also tells us what types of
plants and animals are able to live in the estuary. All
plants and animals have a range of temperatures in
which they thrive and reproduce. For instance, salmon
will only breed at temperatures below 18°F. If the water
in the estuary is outside the normal seasonal temperature
range in which most estuarine organisms can
comfortably live, it is probably an indication that
something is adversely affecting the health of the
estuary.
Reserve (NERR) in California. Elkhorn Slough is one of
the relatively few coastal wetlands remaining in Califor-
nia. The main channel of the slough, which winds inland
nearly seven miles, is flanked by a broad salt marsh
second in size in California only to San Francisco Bay.
The reserve lands also include oak woodlands,
grasslands and freshwater ponds that provide essential
coastal habitats that support a great diversity of native
organisms and migratory animals.
Review of Abiotic Factors
What follows is some basic information about four abi-
otic factors.
pH
pH is a measure of how acidic or basic a solution is. The
pH scale ranges from 0 to 14. Solutions with a pH of
less than 7 are acidic, and those with a pH greater than 7
are basic (or alkaline).
Knowledge of pH is important because most aquatic
organisms are adapted to live in solutions with a pH
between 5.0 and 9.0. The pH in an estuary tends to
remain relatively constant because the chemical
components in seawater resist large changes to pH.
Biological activity, however, may significantly alter pH in
the freshwater portions of the estuary.
pH is actually a measure of the amount of hydrogen ions
in a solution. In fact, some people think of pH as being
the “power of hydrogen.” A lower pH indicates that
there are more free hydrogen ions in the water, which
creates acidic conditions, and a higher pH indicates there
are less free hydrogen ions, which creates basic
conditions. pH is equal to the negative logarithm of the
hydrogen ion activity, meaning that the hydrogen ion
concentration changes tenfold for each number change
in pH unit. Water on the surface of Earth is usually a
little acidic or basic due to both geological and biological
influences.
Through a process called photosynthesis, plants remove
carbon dioxide (CO
2
) from the water and emit oxygen
(O
2
). Since CO
2
becomes carbonic acid when it
dissolves in water, the removal of CO
2
results in a higher
pH, and the water becomes more alkaline, or basic.

3
Life Science Module—Activity 1
Differences in water temperature cause the formation of
distinct, non-mixing layers in water, otherwise known as
stratification, because the density of water changes with
temperature. This stratification leads to chemically and
biologically different regions in water.
Dissolved Oxygen
To survive, fish, crabs, oysters and other aquatic animals
must have sufficient levels of dissolved oxygen (DO) in
the water. The amount of dissolved oxygen in an
estuary’s water is the major factor that determines the
type and abundance of organisms that can live there.
Oxygen enters the water through two natural processes:
(1) diffusion from the atmosphere and (2)
photosynthesis by aquatic plants. The mixing of surface
waters by wind and waves increases the rate at which
oxygen from the air can be dissolved or absorbed into
the water.
DO levels are influenced by temperature and salinity.
The solubility of oxygen, or its ability to dissolve in
water, decreases as the water’s temperature and salinity
increase. Therefore, DO levels in an estuary can also
vary seasonally, with the lowest levels occurring during
the late summer months when temperatures are highest.
Bacteria, fungi, and other decomposer organisms can
reduce DO levels in estuaries because they consume ox-
ygen while breaking down organic matter. Oxygen de-
pletion may occur in estuaries when many plants die and
decompose, or when wastewater with large amounts of
organic material enters the estuary. In some estuaries,
large nutrient inputs, typically from wastewater,
stimulate algal blooms. When the algae die, they begin to
decompose. The process of decomposition depletes the
surrounding water of oxygen and, in severe cases, leads
to hypoxic (very low oxygen) conditions that can kill
aquatic animals. Shallow, well-mixed estuaries are less
susceptible to this phenomenon because wave action
and circulation patterns supply the waters with plentiful
oxygen.
Salinity and Conductivity
Under laboratory conditions, pure water contains only
oxygen and hydrogen atoms, but in the real world, many
substances, like salt, are dissolved in water. Salinity is the
concentration of salt in water, usually measured in parts
per thousand (ppt). The salinity of seawater in the open
ocean is remarkably constant between 30 and 35 ppt.
Salinity in an estuary varies according to one’s location
in the estuary, daily and storm-driven tides, and the vol-
ume of fresh water flowing into the estuary.
Salinity and conductivity are closely related. Both
measure the water’s ability to conduct electricity, which
is a surrogate measure estimating the quantity of salts
dissolved in the water. Conductivity is a more sensitive
measure (parts per million or less) than salinity (parts per
thousand or greater). Pure water is a very poor
conductor of electrical current, but salts such as sodium,
calcium, magnesium, and chloride, dissolved in the water
are in ionic (charged) form and conduct electrical
current. Conductivity, which is the opposite of
resistance, measures the ability of water to conduct
current. A higher conductivity indicates less resistance,
and means that electrical current can flow more easily
through the solution.
In saltwater estuaries, salinity and conductivity levels are
generally highest near the mouth of a river where ocean
water enters, and lowest upstream where freshwater
flows in. Actual salinities vary throughout the tidal cycle,
however, because as the tide rises more ocean water
enters the estuary. In saltwater estuaries, salinity and
conductivity typically decline in the spring when
snowmelt and rain increase the freshwater flow from
streams and groundwater. In freshwater estuaries,
salinity or conductivity is normally the reverse. The
waters of the Great Lakes have a lower salinity that the
streams and rivers flowing into them. Lake water
intrusion due to storm surges or seiches results in lower
salinity near the mouth of the estuary. During storms
and the resulting runoff, both salinity and conductivity
levels usually decrease, as rainwater and the resulting
surface runoff are very low in salts. Although this
decrease is measurable in freshwater estuaries, it does
not have the same ecological impact that it would in a
marine estuary. Salinity and conductivity are frequently
higher during the summer when higher temperatures
increase levels of evaporation in the estuary.
Conductivity and salinity are dependent on many
factors, including geology, precipitation, surface runoff,
and evaporation. Conductivity, because it is a much

4
Life Science Module—Activity 1
more sensitive measurement, is also very temperature
dependent. It increases as water temperature increases
because water becomes less viscous and ions can move
more easily at higher temperatures. Because of this, most
reports of conductivity reference specific conductivity.
Specific conductivity adjusts the conductivity reading to
what it would be if the water were 25°C. This is
important for comparing conductivities from waters
with different temperatures.
Environmental factors that increase conductivity and
salinity include: increased temperature, fertilizers from
agriculture, sewage, road runoff containing automobile
fluids and de-icing salts, and a local geology high in
soluble minerals, such as carbonates. Conductivity and
salinity also increase due to evaporation. The Great Salt
Lake in Utah is an extreme example of how evaporation
can increase salinity. On warm days, the evaporation of
water concentrates the ions that remain behind, resulting
in water with higher conductivity and salinity. Often,
small diurnal fluctuations in conductivity and salinity are
seen as a result of evaporation during the day and
condensation and groundwater recharge at night. In
saltwater estuaries, the influx of ocean water due to
rising tides increases salinity and conductivity within the
estuary.
Estuarine organisms have different tolerances and
responses to salinity changes. Many bottom-dwelling
animals, like oysters and crabs, can tolerate some change
in salinity, but salinities outside an acceptable range will
negatively affect their growth and reproduction, and
ultimately, their survival.
Salinity also affects chemical conditions within the
estuary, particularly levels of dissolved oxygen in the wa-
ter. The amount of oxygen that can dissolve in water, or
solubility, decreases as salinity increases. The solubility
of oxygen in seawater is about 20 percent less than it is
in fresh water at the same temperature.
- Adapted from the NOAA/NOS Estuary Discovery Kit..
URL:http://oceanservice.noaa.gov/education/kits/estuaries/
estuaries10_monitoring.html. Accessed: 2008-07-20.
(Archived by WebCite
®
at http://www.webcitation.org/5ZSbp3Ivp)
Students
Need to work in a computer lab or with a
computer and projector
Copy of the Student Reading 1
Introduction to South Marsh
Copy of the Student Reading 2
Survival in an Estuary
Copy of Student Worksheet
Survival in an Estuary
Copy of Data Sheet
South Marsh at Elkhorn Slough 2004-05
View the SWMP tutorial http://coast.noaa.gov/
swmp/tutorial/tutorial.html
Teachers
Download the PowerPoint presentation entitled
Survival in an Estuary. (To find the presentation
go to the Estuaries.noaa.gov website, choose
the Curriculum tab, search for this lesson by
name and then select the file from the
downloads in the box on the right.)
To find more about these abiotic factors go to
the Estuaries.noaa.gov Web site, choose the
Science and Data tab, click on Data Parameters.
Bookmark the site:
https://coast.noaa.gov/swmp/#/index
Equipment:
Computer lab or
Computer and Projector
Materials

5
Life Science Module—Activity 1
Preparation
Download the PowerPoint presentation entitled
Survival in an Estuary, and prepare to project it in
front of the class.
To find more about these abiotic factors go to the
Estuaries.noaa.gov Web site, choose the Science
and Data tab, click on Data Parameters. Students
will be able select various factors to visually explain
abiotic parameters to them.
If possible, arrange for students to have access to
online data either by obtaining a computer projector
to present the data in front of the whole class or by
arranging for student groups to view the data on
individual computers. On the computer(s),
bookmark the site: <https://coast.noaa.gov/swmp/
#/index>. Static data are also provided in this guide
if arranging computer access is difficult.
Make copies of the Student Reading, Student Worksheet,
and Student Data Sheet. The graphs on the Student
Data Sheet can alternatively be projected in front of
the class.
National Science Education Standards
Content Standard A: Science as Inquiry
A3. Use technology and mathematics to improve
investigations and communications.
A4. Formulate and revise scientific explanations
using logic and evidence.
A6. Communicate and defend a scientific argument.
Content Standard C: Life Science
C4. The interdependence of organisms
C5. Matter, energy, and organization in living sys-
tems
C6. The behavior of organisms
Content Standard E: Science and Technology
E2. Apply and adapt a variety of appropriate
strategies to solve problems
Procedure
Part 1 — The Estuarine Environment
1a. Ask the students what resources and conditions they
need to survive in their environment. They will
probably mention food, water, warm clothes, etc.
They may forget things like oxygen to breathe, and
the right array of vitamins and minerals, amino acids,
and other chemical compounds needed to maintain
good health. Choose an estuarine animal or plant
and ask students to suggest factors such as
temperature that affect conditions in its habitat. List
them on the board. Bring up the water quality
factors used in this activity if students do not include
them:
• temperature
• pH
• salinity
• dissolved oxygen.
For your information, the student worksheet
contains a list of specific conditions necessary for
survival for selected species.
1b. Show students the Data Parameters
sections that deal with SWMP data and water
quality factors.
2. Show the PowerPoint Survival in an Estuary and ask
students to describe the environment they see. Ask
some probing questions as they view the slides:
• What are the water conditions like—deep or
shallow, wide or narrow, salty or fresh?
• What is the biological community like—rich and
abundant, sparse, or in between?
• Have students read the introductory section of
their handout.
3. Have students complete Part 1 of the Student
Worksheet—Survival in an Estuary.

6
Life Science Module—Activity 1
Check for Understanding
1. Direct your students to the Data Graphing Tool on
estuaries.noaa.gov: <https://coast.noaa.gov/swmp/#/
index>. Help students navigate through the site until
they can successfully download trend analysis data for
2005 from one monitoring sta-tion at four other NERR
sites. Encourage them to choose sites both in your
region and in other parts of U.S. coastal areas. OR,
download sample data from four sites and hand them
out to students.
2. Direct students to fill out an Extreme Conditions table
for each site.
3. Have students create graphs comparing parameter
ranges and time between extremes for new sites with
South Marsh data.
4. Discuss with students the patterns they see and ask
them to explain why the ranges and rates of change for
each factor vary at different estuary sites. Or ask them
to write their answers down and collect student work to
serve as a summative evaluation for this activity.
Optional Extension Inquiries
Locate a local water source (pond, river, stream, or
lake) close to your school.
Have students monitor water temperature, pH, salinity,
and DO (if possible) daily or weekly over an extended
period of time.
Direct students to graph their summary data and then
compare their data to the variation of parameters in the
NERR sites featured in this activity.
Discuss with students the differences in water quality
between your local site and that of the NERR sites. Is
your local water source habitable for all animal species
featured in this activity? What could be done to improve
the water quality in your local water source?
4. Have students read the Student Reading—Survival in an
Estuary and Student Reading—Introduction to South
Marsh.
Part 2 — Survival Changes: Abiotic Factors that
Affect Life
5. Go over the graphs on the Student Data Sheet—South
Marsh at Elkhorn Slough 2004-5, discussing the units
on the axes: the y-axis of each graph is different; the
x-axis of each graph represents one year of time at
South Marsh in the Elkhorn Slough.
6. Have students complete Part 2 of the Student
Worksheet—Survival in an Estuary.
7. Review and discuss the Part 2 tasks and questions.
Part 3 — Surviving in an Estuary: Extreme
Conditions
8. Use the following procedure to have students access
or display in front of the class the graphs that show
the actual values, measured by buoy, of the four
factors: water temperature, pH, salinity, and
dissolved oxygen.
Go to <https://coast.noaa.gov/swmp/#/
index> to find the graphing tool and click on
the tutorial to learn how to generate a graph.
Choose the type of data: water for water quality
parameters and then click on “CA, Elkhorn
Slough, South Marsh”
Project the buoy data on the screen and assist
students in interpreting the readings.
9. Have students complete Part 3 of the Student
Worksheet—Survival in an Estuary.
Teacher Notes:
To find whether a station will have today’s data we recommend
checking this link first: https://coast.noaa.gov/swmp/#/index
and select the station you want to see data for.
If you cannot access today’s gauge data, use data for 10/4/07. You
will need to choose, at minimum, one day’s worth of data. You may
want to increase the amount of data that students analyze and
compare by adding several more days, months or years’ worth of
data.
Timestamp: 10/04/2007 06:15
Water Temp: 17.1 C
Percent Saturation: 66.8 %
Turbidity: 5 NTU
Specific Conductivity: 47.98
Salinity: 31.3 ppt
Dissolved Oxygen: 5.3 mg/l
Depth: 1.72 meters
pH: 8.2 units the student
handouts section.

7
Life Science Module—Activity 1
1a. Why is it important to monitor abiotic factors in estuarine environments?
Answer: It is important to monitor parameters such as pH, temperature, salinity, and DO because each of these factors must re-
main within a certain range to ensure the survival of species living in the estuary. Each of these parameters can exceed their normal
range when either natural (storms, floods) or human-caused events (runoff from farms, factories, power plants, sewage treatment facil-
ities) occur.
1b. Based on your observations of the images, describe the environment of species living in an estuary. Consider
factors such as temperature, water flow, salinity, and weather to name a few.
Answer: Estuaries are complex environments in which diverse species exist or vanish depending on physical and chemical factors.
The environment of South Marsh is governed by large swings of temperature and other factors due to seasonal changes. Student an-
swers about their organism will vary.
1c. How is surviving in an estuary different than surviving in a forest, a desert, or in the open ocean?
Answer: Surviving in an estuary is difficult. In an estuary, environmental factors can change rapidly. Conditions in estuaries vary
more than in many other types of habitats. Dramatic changes in pH, salinity, and temperature occur frequently and regularly in
estuaries. In deserts or the open ocean, conditions are more stable and changes usually take place more slowly.
2. Choose one animal that was highlighted in the images of Part 1. What strategies and adaptations do you think
your chosen aquatic species uses to cope with changing abiotic conditions in South Marsh?
Answer: Answers will vary. Hibernation might be mentioned as a strategy to cope with cold, wintry conditions. Some plants such as
cordgrass have special filters in their root system that removes salt from the water it absorbs in from the saltmarsh. Bivalves like
mussels, clams, and oysters close their shells during low tide and stop feeding and change their method of respiration until they are
again covered with seawater. Some aquatic species can migrate to areas with more favorable conditions and move up river or down
depending on the salinity at a particular time.
3a. After examining the range of tolerance information for five estuarine species, which of the five organisms do
you think would thrive in the abiotic conditions of South Marsh today? Which could survive over the course of
a year?
Answer: Answers will vary depending on the current abiotic data.
3b. Review the two-year data set for each abiotic factor in this activity. Choose whether each of the
five species on your list is:
i) likely to survive and live in South Marsh
ii) might do fairly well
iii) doubtful to survive given the long-term environmental conditions of South Marsh.
Teacher Worksheet with Answers
Activity 1: Survival in an Estuary

Life Science Module—Activity 1
8
Explain your reasoning for each species.
Answer:
oysters = Salinity of the water is uniformly too high for oysters.
clams = Water temperatures are too cold for clams to spawn.
alewife = DO levels are on the low side.
blue crab = Yes, all factors are within the survival limits of a blue crab.
cohoe salmon = DO somewhat low for salmon, average temperature is too high even though the salinity is good.

Life Science Module—Activity 1
9
South Marsh is part of the Elkhorn Slough National
Estuarine Research Reserve in California. The South
Marsh Complex is located on the southeastern side of
Elkhorn Slough. The entire complex is approximately
415 acres in size. Mudflat areas with some subtidal
creeks, fringing tidal marsh, and created tidal marsh
islands dominate the main areas.
Elkhorn Slough is one of the relatively few coastal
wetlands remaining in California. The main channel of
the slough, which winds inland nearly seven miles, is
flanked by a broad salt marsh second in size in
California only to San Francisco Bay.
The reserve lands also include oak woodlands,
grasslands and freshwater ponds that provide essential
coastal habitats that support a great diversity of native
organisms and migratory animals.
Student Reading—1
Activity 1: Introduction to South Marsh
Figure 1. Satellite view of Elkhorn Slough NERR

Life Science Module—Activity 1
10
More than 400 species of invertebrates, 80 species of
fish, and 200 species of birds have been identified in
Elkhorn Slough. The channels and tidal creeks of the
slough are nurseries for many species of fish.
At least six threatened or endangered species utilize the
slough or its surrounding uplands, including peregrine
falcons, Santa Cruz long-toed salamanders, California
red-legged frogs, brown pelicans, least terns, and sea
otters.
Additionally, the slough is on the Pacific Flyway,
providing an important feeding and resting ground for
many types of migrating waterfowl and shorebirds. The
slough and surrounding habitat are renowned for their
outstanding birding opportunities.
Many habitat types are located within a short distance
from the slough. Upland hills with oak, pine, eucalyp-
tus, grassland and maritime chaparral surround the
slough. Several thousand acres of salt marsh, tidal flats
and open water comprise the main channel of the
slough. Beach and sand dunes separate the estuary from
Monterey bay. Riparian habitat is also found on the re-
serve. Agricultural lands and residential areas border the
reserve. The close proximity of these varied habitats
supports a remarkable diversity of plant and animal spe-
cies in a relatively small area.
— Adapted from http://nerrs.noaa.gov/ElkhornSlough/welcome.html
Figure 2. South Marsh is in the foreground of this image.
Figure 3. The Elkhorn Slough National Estuarine Research Reserve encom-
passes only 1400 acres of marsh and upland habitat in the top right corner of
this image. The rest of Elkhorn Slough and the surrounding lands are owned
and managed by a variety of other individuals and entities including the
California Department of Fish and Game, The Nature Conservancy, the Elkhorn
Slough Foundation, the Moss Landing Harbor District, and the
Monterey Bay National Marine Sanctuary

Life Science Module—Activity 1
11
Figure 4. Vegetation map of the Elkhorn Slough watershed courtesy
of the Elkhorn Slough Foundation.

12
Life Science Module—Activity 1
An estuary is a partially enclosed body of water where
two different bodies of water meet and mix such as
fresh water from rivers or streams and salt water from
the ocean, or fresh water from rivers or streams and
chemically distinct water of the Great Lakes. In estuar-
ies, water levels are affected by lunar or storm driven
tides. In fresh water, the concentration of salts, or sa-
linity, is nearly zero. The salinity of water in the ocean
averages about 35 parts per thousand (ppt). The mix-
ture of sea water and fresh water in estuaries is called
brackish water.
Estuaries are transitional areas that connect the land
and the sea, as well as freshwater and saltwater habitats.
The daily tides (the regular rise and fall of the sea’s sur-
face) are a major influence on many of these dynamic
environments. Most areas of the Earth experience two
high and two low tides each day. Some areas, like the
Gulf of Mexico, have only one high and one low tide
each day. The tidal pattern in an estuary depends on its
geographic location, the shape of the coastline and
ocean floor, the depth of the water, local winds, and
any restrictions to water flow. For example, tides at the
end of a long, narrow inlet might be heightened be-
cause a large volume of water is being forced into a
very small space. However, the tidal change in wetlands
composed of broad mud flats might appear to be ra-
ther small.
While strongly affected by tides and tidal cycles, many
estuaries are protected from the full force of ocean
waves, winds, and storms by reefs, barrier islands, or
fingers of land, mud, or sand that surround them. The
characteristics of each estuary depend upon the local
climate, freshwater input, tidal patterns, and currents.
Truly, no two estuaries are the same.
Survival for any species, regardless of its environment,
depends on the ability to adapt to changing conditions.
Humans can go inside to get warm on a freezing cold
day or put on a heavy coat and gloves. Or if the water
main breaks or if the well runs dry, we can hop in our
cars and obtain water from another source like a neigh-
bor or local store. For plants and animals that live in an
aquatic environment, adaptation is sometimes much
more difficult. And for every species that spends most
of its time in water, sudden changes in the environ-
ment, whether caused by natural agents (storms) or hu-
man intervention (pollutants), can spell disaster and
lead to the death of many members of the aquatic com-
munity.
In estuaries, all plant and animal species live in a transi-
tion zone where fresh and salt water meet. Factors that
cause change in estuarine environment fall into two
categories: abiotic and biotic. Abiotic factors are those
that occur in physical environment such as amount of
sunlight, climate, and the geology of the area. Biotic
factors are those that deal with the organism and other
organisms they share their environment with, including
their interaction, wastes, disease and predation.
To measure changes in the physical environment, biol-
ogists use factors that relate to natural processes or hu-
man actions. These include:
pH
Scientists use pH as an indicator of whether water is
acidic or basic. pH is measured on a scale of 1 to 14,
where numbers less than 7 are increasingly acidic and
numbers greater than 7 are increasingly basic. Distilled
water has a pH of 7 and is said to be neutral. Water on
the surface of Earth is usually a little
acidic or basic due to both geological
and biological influences.
pH is actually a measure of the amount of
hydrogen ions in solution. In fact, some
people think of pH as being the “power
Student Reading—2
Activity 1: Survival in an Estuary

13
Life Science Module—Activity 1
Figure 5. Barrier beach closed Figure 6. Barrier beach open
of hydrogen.” A lower pH indicates that there are more
free hydrogen ions in the water, which creates acidic
conditions, and a higher pH indicates there are less free
hydrogen ions, which creates basic conditions. pH is
equal to the negative logarithm of the hydrogen ion
activity, meaning that the hydrogen ion concentration
changes tenfold for each number change in pH unit.
Water on the surface of Earth is usually a little acidic or
basic due to both geological and biological influences.
All aquatic organisms have a pH range to which they
are adapted. Outside of this range, critical biological
processes may be disrupted, leading to stress and death.
Most organisms cannot live below a pH of 5 or above a
pH of 9. Additionally, pH is used to monitor safe water
conditions. Once the background range of pH has
been established, a rise or fall in pH may indicate the
release of a chemical pollutant or an increase in acid
rain. Additionally, pH affects the solubility, biological
availability, and toxicity of many substances. For exam-
ple, most metals are more soluble, and often more tox-
ic, at lower pH values.
Temperature
Temperature is a measure of kinetic energy, or energy
of motion. Increasing water temperature indicates in-
creasing energy, or motion of water molecules and sub-
stances dissolved in the water. Temperature is a critical
factor for survival in any environment. Organisms that
live in water are particularly sensitive to sudden chang-
es in temperature.
The Celsius temperature scale is used worldwide to
measure temperature. Temperature has a significant
impact on water density. Water density is greatest at 4
degrees Celsius, meaning that water at higher or lower
temperatures will float on top of water at or near 4º C.
This is why ice floats on water, and warm water floats
over cooler water. Differences in water temperature
cause the formation of distinct, non-mixing layers in
water, otherwise known as stratification. This stratifica-
tion leads to chemically and biologically different re-
gions in water.
Salinity and Conductivity
Salinity and conductivity are measures of the dissolved
salts in water. Salinity is usually described using units of
parts per thousand or ppt. A salinity of 20 ppt means
that there are 20 grams of salt in each 1000 grams of
water. Because it is impractical to routinely determine
the total amount of salts dissolved in water, a surrogate
measure—the ability of the water to conduct electrici-
ty—is made for determining both conductivity and sa-
linity. All aquatic life in an estuary must be able to sur-
vive changes in salinity. All plants and animals have a
range of salinity to which they are adapted. Outside of
this range, they will be unable to function and may die.
Salinity and conductivity are closely related. Conductiv-
ity and salinity are measures of what is dissolved in the
water. Pure water is a very poor conductor of electrical

14
Life Science Module—Activity 1
current, but salts dissolved in the water are in ionic
(charged form) and conduct electrical current. Conduc-
tivity, which is the opposite of resistance, measures the
ability of water to conduct current. A higher conductiv-
ity indicates less resistance, and means that electrical
current can flow more easily through the solution. Be-
cause dissolved salts conduct current, conductivity in-
creases as salinity increases. Common salts in water
that conduct electrical current include sodium, chloride,
calcium, and magnesium.
Salinity affects the ability of water to hold oxygen, and
seawater holds approximately 20% less oxygen than
freshwater. Many chemical reactions that determine the
concentration of nutrients and metals in the water are
influenced by salinity. The conductivity and salinity of
seawater is very high while these parameters are com-
paratively low in tributaries and rivers. Freshwater lakes
typically have conductivities and salinities even lower
than those of inland streams. This is because inland
streams pick up salts from rocks, soils, and roads as
they flow over the landscape.
Many chemical reactions that determine the concentra-
tion of nutrients and metals in the water are influenced
by salinity. For instance, salinity and conductivity affect
the ability of particles to flocculate, or stick together,
which is important in determining turbidity levels and
sedimentation rates. Salinity also increases the density
of water, with seawater being heavier than freshwater.
This density difference inhibits mixing. In fact, conduc-
tivity and salinity serve as excellent indicators of mixing
between inland water and sea or lake water, and they
are particularly useful in indicating pollution events or
trends in freshwater. For example, an overdose of ferti-
lizers or the application of road salt will cause spikes in
conductivity and salinity.
Conductivity and salinity are dependent on many fac-
tors, including geology, precipitation, surface runoff,
and evaporation. Since conductivity is a much more
sensitive measurement than salinity, it is more impacted
by changes in temperature. Conductivity increases as
water temperature increases because water becomes
less viscous and ions can move more easily at higher
temperatures. Because of this, most reports of conduc-
tivity reference specific conductivity. Specific conduc-
tivity adjusts the conductivity reading to what it would
be if the water was 25°C. This is important for compar-
ing conductivities from waters with different tempera-
tures.
Dissolved Oxygen
Dissolved oxygen (DO) is the amount of oxygen gas
that is dissolved in a sample of water. DO is usually
measured in units of milligrams per liter (mg/L). Just as
we need air to breathe, aquatic plants and animals need
dissolved oxygen to live. Dissolved oxygen is used for
respiration, which is the process by which organisms
gain energy by breaking down carbon compounds,
such as sugars. Dissolved oxygen is also essential for
decomposition, which is a type of respiration in which
bacteria break down organic materials for energy. De-
composition is an important process that recycles nu-
trients and removes organic materials such as dead veg-
etation from our waterways. Because dissolved oxygen
is required for aquatic life, balancing the sources and
sinks of dissolved oxygen is essential in maintaining a
healthy ecosystem.
The concentration of dissolved oxygen in water is de-
pendent on a number of interrelated factors, including
biological factors, such as the rates of photosynthesis
and respiration, and physical and chemical factors, such
as temperature, salinity, and air pressure.
Dissolved oxygen enters the water by diffusion from
the air and as a byproduct of photosynthesis. Diffusion
from the air occurs very quickly in turbulent, shallow
water or under windy conditions. The amount of oxy-
gen that can dissolve in water is dependent on water
temperature, salinity, and air pressure. As temperature
and salinity increase, and pressure decreases, the
amount of oxygen that can be dissolved in water de-
creases. Cold water holds more dissolved oxygen than
warm water, and water at sea level holds more dis-
solved oxygen than water at high altitudes. Seawater
holds approximately 20% less oxygen than freshwater
at the same temperature and altitude.
— Adapted from NOAA’s National Ocean Service Estuaries Discovery Kit

15
Life Science Module—Activity 1
Student Worksheet
Activity 1: Survival in an Estuary
Student Name:
Procedure
Part 1 — The Estuarine Environment
You will be shown a number of images of estuaries. If you were a (specific) animal or plant living in an (specific loca-
tion) estuary, what factors seen in these images might influence whether you survive or not? Take notes as the images
are shown and then answer the following questions.
1a. Why is it important to monitor abiotic factors in estuarine environments?
1b. Based on your observations of the images, describe the environment of species living in an estuary. Consider fac-
tors such as temperature, water flow, salinity, and weather to name a few.
1c. How is surviving in an estuary different than surviving in a forest, a desert, or in the open ocean?

Life Science Module—Activity 1
16
Part 2 — Surviving Changes: Abiotic Factors that Affect Life
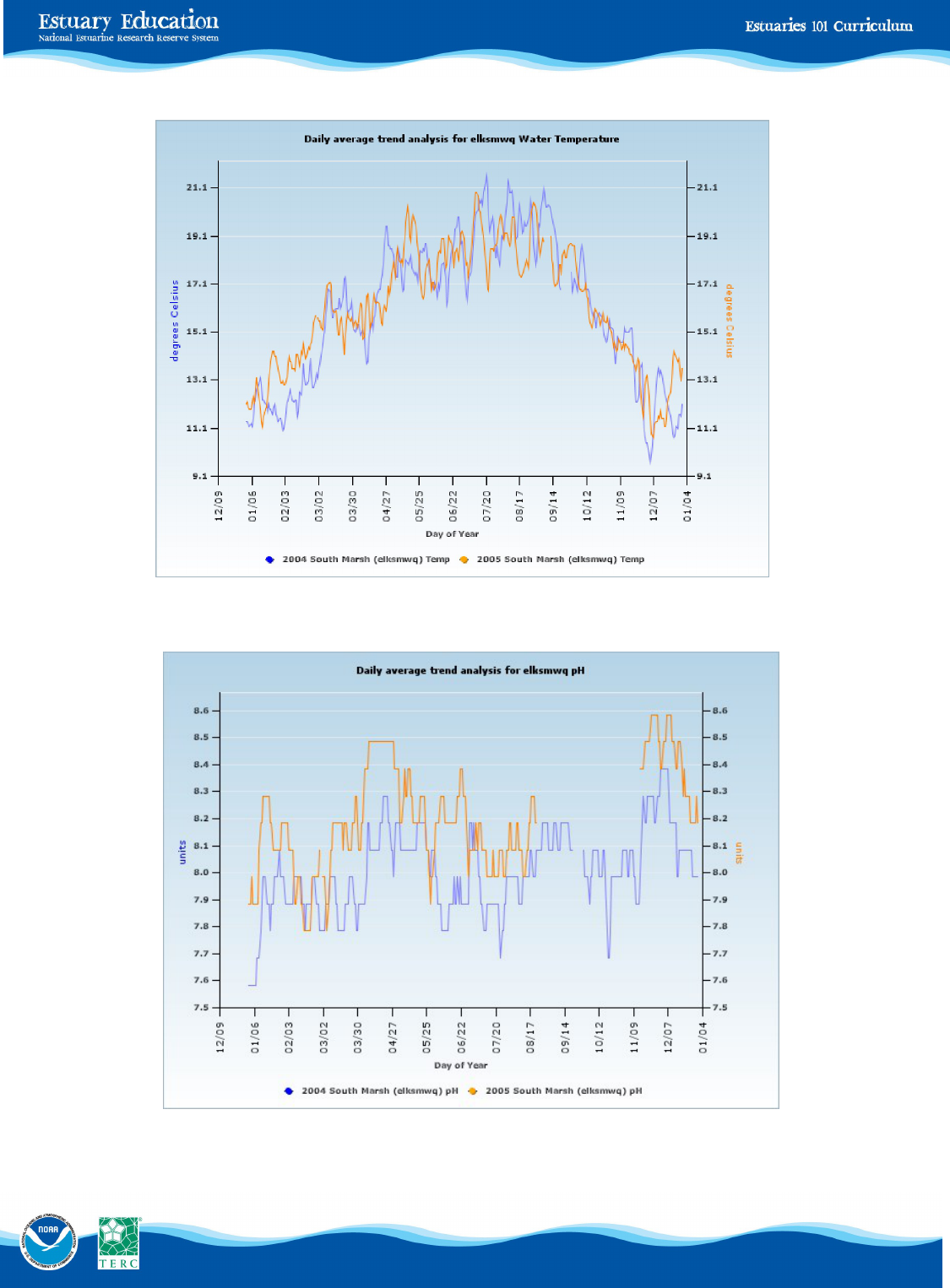
You will investigate two years’ worth of graphical data that describe four abiotic factors affecting the survival of
aquatic species at South Marsh in the Elkhorn Slough.
For each graph on the Student Data Sheet—South Marsh at Elkhorn Slough 2004-5, determine the lowest and highest val-
ue of each abiotic factor. Then determine the approximate time (in days) that elapsed between these two measure-
ments.
Extreme Conditions at South Marsh Table
2004 2005
Factor High Low Time Between High Low Time Between
temperature ___________________________________________________________________
pH __________________________________________________________________________
salinity _______________________________________________________________________
dissolved oxygen _______________________________________________________________
Next, find the range for each factor (high value - low value) for 2004 and 2005.
2. Choose one animal that was highlighted in the images in Part 1. What strategies and adaptations do you think
your chosen aquatic species uses to cope with changing abiotic conditions in South Marsh?

Life Science Module—Activity 1
17
Part 3 — Surviving in an Estuary: Extreme Conditions
You will explore the actual values for each abiotic factor on a specific day. Your teacher will project the buoy readings
for today's date or supply a hardcopy sheet with data for another day.
Record the date your data was gathered.
date ______________________________________________________________________
Record the values for temperature, salinity, dissolved oxygen, and pH.
temperature ________________________________________________________________
pH _______________________________________________________________________
salinity ____________________________________________________________________
dissolved oxygen ____________________________________________________________
Consult the list of Limits of Tolerance to Environmental Factors for Selected Organisms for the animals, and answer the follow-
ing questions.
3a. After examining the range of tolerance information for five estuarine species, which of the five organisms do
you think would thrive in the abiotic conditions of South Marsh today?

18
Life Science Module—Activity 1
3b. Review the two-year data set for each abiotic factor in this activity. Choose whether each of the five species on
your list is:
i) likely to survive and live in South Marsh
ii) might do fairly well
iii) doubtful to survive given the long term environmental conditions of South Marsh.
Explain your reasoning for each species.
Limits of Tolerance to Environmental Factors for Selected Organisms
Oysters
Grow best in water with a salinity of 12 ppt and
above, perish if salinity is below 5 ppt or above 25 ppt
Spawn only when the water temperature hits 18°C for
four hours
Spawn much more prevalent when salinity is over 20
ppt
Need a DO level of around 4 mg/l
Best growth when pH is between 7.5 and 8.5
Clams
Grow best when the water salinity is above 15 ppt
Spawn only when the water temperature hits 24°C for
four hours
Clam eggs die when the salinity is below 20 ppt
Need a DO level of around 4 mg/l
Optimal growth occurs between 10 and 25°C
Alewife
Adult and juvenile fish need a DO level of at least 3.6
mg/l
Alewife eggs and larvae need a DO level of 5 mg/l or
more
Must have a pH higher than 5 but less than 9
Blue Crab
Needs a DO level of 3 mg/l or more for survival, opti-
mal at 5 mg/l
Thrives if pH is between 6.8 and 8.2
Coho Salmon
Like a DO level of 6 mg/l or higher
Require a salinity of greater than 15 ppt
Prefer temperatures between 4° and 20°C, do best at
13°C
Spawn only when temperature is 18°C or higher
Newly hatched salmon need a DO level of at least 5
mg/l to survive
pH of 4.0 or lower or higher than 9 is lethal for salmon

19
Life Science Module—Activity 1
Student Data Sheet
Activity 1: South Marsh at Elkhorn Slough 2004
Salinity
Dissolved Oxygen
Figure 8.
DO: South Marsh
Figure 7.
Salinity: South Marsh
20
Life Science Module—Activity 1
pH
Figure 9.
Water temperature:
South Marsh
Water Temperature
Figure 10.
pH: South Marsh
