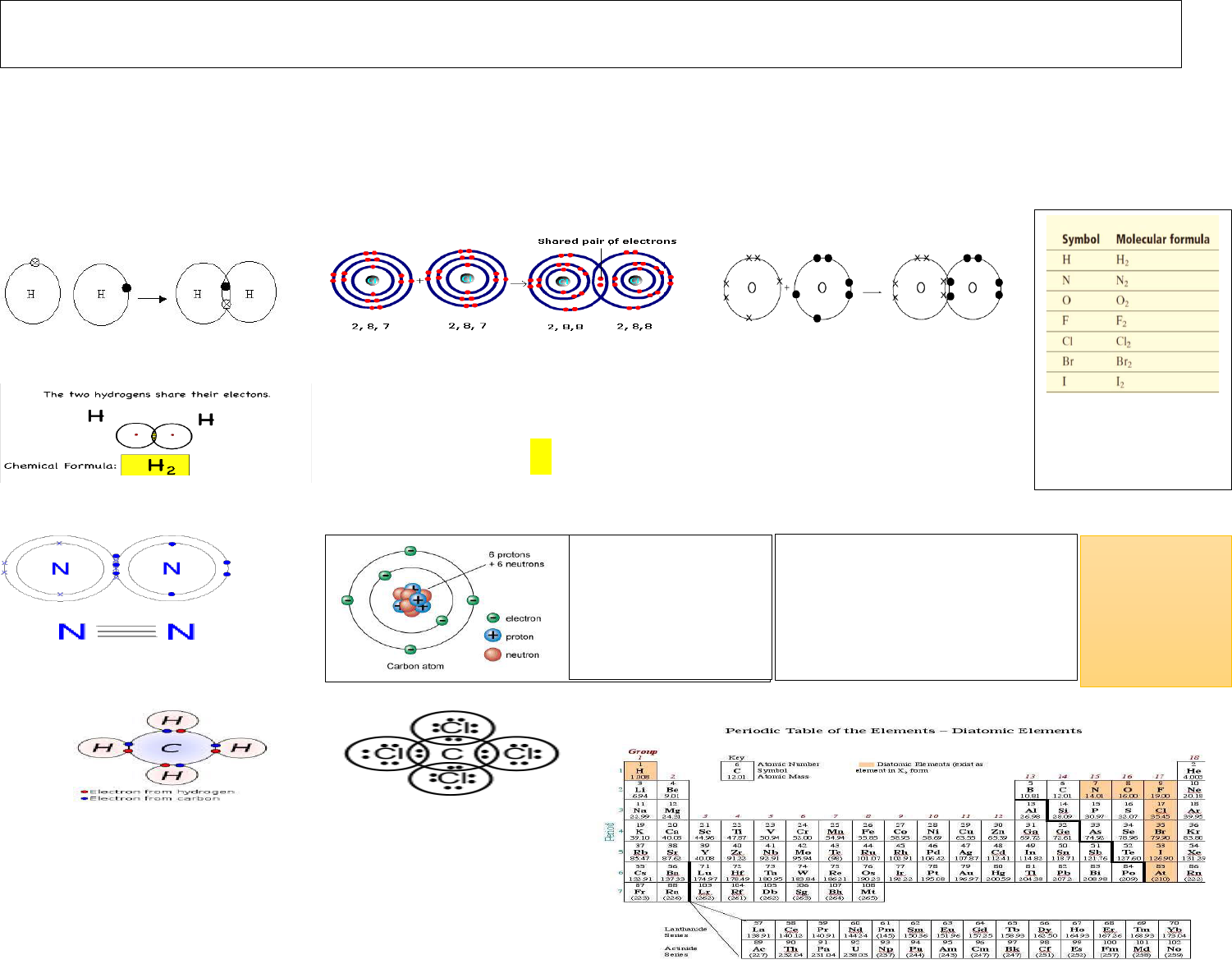
A bond is made up of two electrons. A Covalent Bonds is formed by equal sharing of electrons. That is, if two atoms wish to form a covalent bond, then each atom contributes one electron for
the bond and they thus form a covalent bond in between them. For example, two Hydrogen atoms form a covalent bond to form a hydrogen molecule, H
2
– see Figure 1 below). The covalent
bond in hydrogen is a single bond. Similarly, the covalent bond in chlorine molecule is a single bond (see Figure 2 below). If atoms can share two electrons each, then they form a double bond as
in Oxygen (see Figure 3 below). If atoms can share three electrons each, then they can from a Triple bond as in Nitrogen Molecule (See Figure 4 below). Covalent bond can vary in strength
depending on whether the bond is single, double, or triple; a triple bond is stronger than a double bond, and a double bond is stronger than a single bond. Remember that always valence electrons
are shared in covalent bonding. We use the electronic dot structure to explain covalent bonding. Covalent is generally formed between nonmetals. And atoms of the same element can form
covalent bond – For example, we have Eight elements that form the self- covalent bond to form the Diatomic Molecules. They are Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine,
Iodine, and Astatine.
Hydrogen atoms Hydrogen Molecule Chlorine atoms Chlorine Molecule Oxygen atoms Oxygen molecule
Figure 1 Figure 2 Figure 3
Figure 4
Methane Carbon tetrachloride
CH
4
CCl
4
Single Bond Single Bond Double Bond
Oxygen atoms share two electrons
and form a double bond in the
oxygen molecule, O
2
O=O
Chlorine atoms share one electrons
and form a single bond in the
chlorine molecule, Cl
2
Cl—Cl
H—H
Carbon is an important
element that forms
covalent bonds. Let us
look at the atomic
structure of carbon
Carbon has four valence electrons.
Therefore, it has some interesting
bonding features. It can form a single
bond as in Methane or Carbon
tetrachloride.
Pure and Polar Covalent Bond: Dr. R: Name:-------------------------------------------------; Period:-------Date:------
Standard: SPS1b: Compare and contrast ionic and covalent bonds in terms of electron movement.
At At
2
See the Periodic Table
Below
Covalent Bond is
formed by
elements, which
cannot lose or
gain electron
Daily Formative Assessment: Fill in the Blanks
1. A ------------------ Bond is formed when elements cannot lose or gain electron.
2. A Covalent Bond is formed by ----------- ---------- of the Valence electrons
3. Covalent bond is generally formed between -----------------.
4. Atoms of the ----------element can form covalent bond. For example, all the ------------
-Elements are formed by covalent bond.
The diatomic elements are:
5. Covalent bond can vary in --------------depending on whether the bond is ----------------
------------, or ----------------;
a triple bond is ------------ than a -------- bond, and a ---------- bond is stronger than a --
----------- bond.
6. Remember that always only ---------- electrons are shared in covalent bonding.
7. We use the ------------ dot structure to explain covalent bonding.
Daily Formative Assessment
Draw the Covalent bond of Hydrogen, Nitrogen, and Oxygen molecules
Daily Formative Assessment
1. Draw the Covalent bond of Methane and Carbon tetrachloride
A covalent Bond is of two types: Pure Covalent Bond or Polar Covalent Bond
Covalent Bond
Pure covalent Bond
Polar Covalent Bond
Pure covalent compounds are also called as non-polar
compounds.
2. How many types of covalent bonds are there? What are they?
3. What is the other name for Pure Covalent compound?
Slightly Polar Strongly Polar

If the two atoms connected by a covalent bond are the same or have same of close by electronegativity values, then such a covalent bond is called Pure Covalent
Bond. Electronegatiivty is the pulling power of a bonded over a bonded pair of electrons.
If the two atoms are same or similar in electronegativity, then they are going to pull the
two electrons of the bond with equal force and the bond will remain at the center. That is
the bond will be equidistant from both the atoms. Therefore, both the atoms will remain
neutral like atoms. So a Pure covalent bond connects neutral atoms and no charges are
formed. Hydrogen, Chlorine, Carbon dioxide, Methane, and Carbon tetrachloride are
examples of pure covalent compounds
If the two atoms are different and have different
electronegativity values, then they are going to pull the two
electrons of the bond with unequal force and the bond will not
remain at the center. It will move to one side toward one of the
two atoms. That is the bond is closer to one atom and farther
than the other atom.
Because of the disposition of the electrons of the bond, the
atoms cannot remain neutral, but would become polarized. That
is they will acquire some partial charges; the atom to which bond
has moved closer will acquire partial negative charge and the
atom to which the bond is farther will acquire a partial negative
charge.
Hydrogen chloride is a good example of a Polar Covalent
compound.
See the bottom box on the right and look at the
electronegativities of elements in the Electronegativity Periodic
Table.
Hydrogen atom and chlorine atom combine to form hydrogen chloride. The bond is a
covalent bond since each of them equally share the electrons of the bond. However,
chlorine is more electronegative than hydrogen. Therefore, it pulls the bonded electrons
toward itself. As a result, the bond becomes a Polar Covalent Bond and a Dipole is
formed. So, the Hydrogen Chloride molecule is a Dipole.
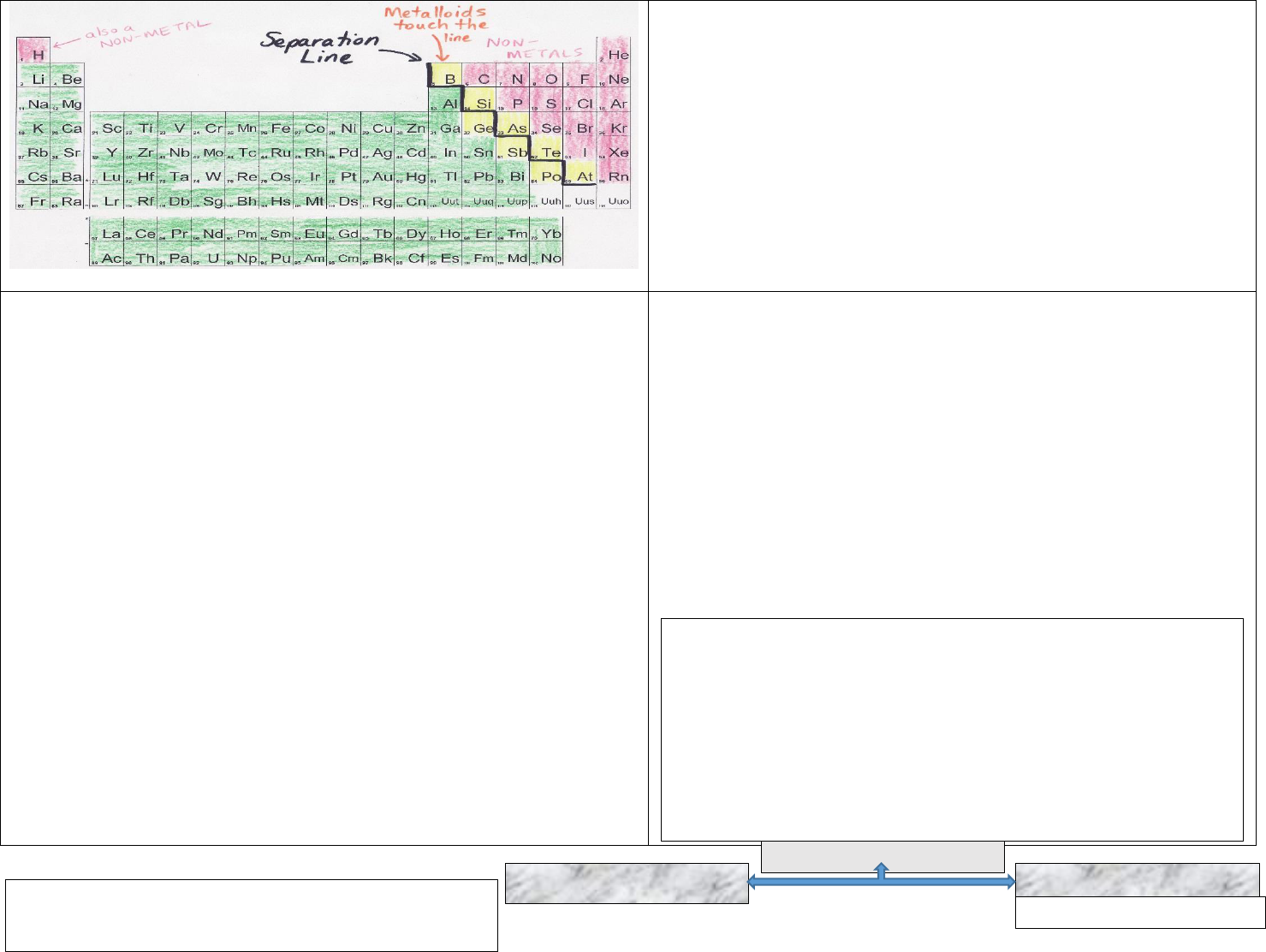
Electronegativity (EN) is given in numeric values. See Electronegativity
Periodic Table below: The following rule is used to determine whether a
bond is Pure Covalent Bond or a Polar Covalent Bond:
1. If the electronegativity difference (usually called ΔEN) is less
than 0.5, then the bond is nonpolar covalent.
2. If the ΔEN is between 0.5 and 1.6, the bond is considered polar
covalent
Electronegativity difference between hydrogen and
chlorine
Hydrogen has an electronegativity of 2.1, and
chlorine has an electronegativity of 3.0. The
electron pair that is bonding HCl together shifts
toward the chlorine atom because it has a larger
electronegativity value.
Water is a polar covalent compound. See Explanation on Page 5
Note that the bond is equidistant from the two atoms in Pure Covalent Bond. In
the Polar Covalent Bond, the bond is closer to one atom than the other atom.

Covalent Bond Interim Review
1. Covalent Bond is formed when elements cannot lose or gain electron
2. A Covalent Bond is formed by equal haring of the Valence electrons
3. Covalent bond is generally formed between nonmetals.
4. Atoms of the same element can form covalent bond. For example all the Diatomic
Elements are formed by covalent bond.
The diatomic elements are: Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine,
Iodine, and Astatine,
5. Covalent bond can vary in strength depending on whether the bond is single, double,
or triple;
a triple bond is stronger than a double bond, and a double bond is stronger than a
single bond.
6. Remember that always valence electrons are shared in covalent bonding.
7. We use the electronic dot structure to explain covalent bonding.
8. Electronegativity determines whether the covalent bond formed is a Pure Covalent
Bond or a Polar Covalent Bond.
9. A Pure covalent bond is formed when the electronegativity values between the atoms
is zero or less than 0.5
10. A polar covalent bond is formed when the electronegativity difference between the
atoms is between 0.5 and 1.6.
11. In the Periodic Table, the Electronegativity increases as you move from Left
to Right and it decreases as you move from top to bottom.
Daily Formative Assessment: Fill in the blanks
1. Covalent Bond is formed when elements cannot ------- or -------electron
2. A Covalent Bond is formed by --------- ---------- of the Valence electrons
3. Covalent bond is generally formed between --------------------.
4. ---------- of the ------- ------------ can form covalent bond. For example all the ----------
--- --------------- are formed by covalent bond.
The diatomic elements are: -----------------------------------------------------------------------
--------------------------------------------------------------------------------------
5. Covalent bond can vary in ---------- depending on whether the bond is single, -------
---------, or --------------; a ---------- bond is stronger than a -------- bond, and a ---------
- bond is stronger than a ----------- bond.
6. Remember that always valence electrons are --------- in covalent bonding.
7. We use the --------------- -------- ------------ to explain covalent bonding.
8. -------------------- determines whether the covalent bond formed is a -------- Covalent
Bond or a ------------- Covalent Bond.
9. A ------- covalent bond is formed when the electronegativity values between the
atoms is --------- or less than -------.
10. A -------- covalent bond is formed when the electronegativity difference between
the atoms is between ------- and ---------.
11. In the Periodic Table, the Electronegativity ------------------- as you move from Left to
Right and it -------------------------- as you move from top to bottom.
Properties of Covalent Compounds
Pure Covalent Compounds
Polar Covalent Compounds
Insoluble or very slightly soluble in
water
Soluble in water
Low Melting Point
Slightly Higher Melting Point
Low Boiling Point
Slightly Higher Boiling Point
Low vapor pressure
Somewhat higher vapor pressure
Strong odor
Mild odor
Mostly Liquids or gas or vapors;
some or very low melting waxy
substances.
Mostly Liquids and some are semisolids
like cheese. Rarely, some are gases like
carbon dioxide.
Do not conduct electricity
Do not conduct electricity by
themselves, and can conduct electricity
when dissolved in water.
Examples:
Butter, camphor, Iodine, Wax,
Hydrogen, Oxygen, Nitrogen
Examples
Cheese, Carbon dioxide, Water, Alcohol,
pure vinegar
Cheese has a lot of intermolecular forces because it is a protein; butter has no
strong intermolecular forces because it is a fat.
Daily Formative Assessment
Take Covalent Bonding Quiz at Softschools Chemistry Quiz: Chemical
Bonding: II Covalent Bonding
On a Separate sheet of paper draw Polar covalent bond in Hydrogen
Chloride and water.
Intermolecular Forces Determine Melting and Boiling Points
Pure covalent molecules are internally very strongly bonded to each other
atom because of equal sharing of electrons. It is because of this strong
internal bonding, covalent molecules tend to behave in isolated way and have
weak connections to other molecules. Therefore, they need very little energy
to move and show low melting and boiling points.
Polar covalent molecules are dipoles and therefore they tend to have strong
intermolecular forces – the positive pole of one molecule can attract the
negative pole of another molecule and they tend to stay together. We have to
supply some energy to separate the molecules and make them move.
Therefore, polar covalent molecules have higher melting and boiling points
tahtn the pure covalent compounds.

O=C=O
O=C=O
X Electron from Carbon Two Double Bonds
• Electron from Oxygen
Behaves like a Pure
Covalent Molecule
Carbon dioxide is a Polar Covalent Molecule but it
behaves like a Pure Covalent Molecule
The situation of Carbon dioxide is unique. Carbon equally shares two
electrons with each oxygen atom. Therefore, there it forms one double
bond with one oxygen and another double bond with the second oxygen.
Carbon dioxide is actually a polar covalent bond because of the
electronegativity difference between carbon and oxygen. However, the
two oxygen atoms are on either side of carbon and they pull the bonded
pairs of electrons equally in opposite directions. Therefore, there is no net
effect of the pull. This means that although carbon dioxide is a polar
covalent molecule, it behaves normally like the pure covalent molecule.
Water is a Polar Covalent Molecule
Electronegativity Values: H = 2.1; O = 3.5. The difference is 3.5-2.5 = 1.4. Since the
difference in electronegativity is in the range 0.5 to 1.6, the bond is a Polar Covalent
Bond. Unlike carbon dioxide the pull is toward the center, that is, toward oxygen.
Therefore, the pulling power of oxygen is double and water is really strongly polar
and it results in water molecule being a dipole.
In other words, the covalent bond in water is considerably polarized and there is
somewhat appreciable amount of partial charges in water. It is because of this
nature, water is able to readily dissolve all ionic compounds and also other polar
covalent compounds like carbon dioxide. As we are aware the drinking soda is a
solution of carbon dioxide in water – it is actually carbonic acid – a weak compound
between water and carbon dioxide:
Electronegativity Values: C = 2.5; O = 3.5. The
difference is 3.5-2.5 = 1.0. Since the difference
in electronegativity is in the range 0.5 to 1.6, the
bond is a Polar Covalent Bond.
Two different charges
on the molecule makes
water a Dipole. A dipole
is a polar covalent
molecules with partial
positive and partial
negative charges.
Water
molecule
is a
dipole
Carbon
dioxide
molecule is a
dipole

Pure or Non-polar compounds dissolve in Non-Polar Compounds – The like likes the like
1. We saw that Pure or Non-polar or covalent molecules will not dissolve in water. The reason is water is a polar solvent and it will not let in a
mon-polar substance. Even if water dissolves any non-polar molecule, it will do so only to a very small extent. For example, the solubility of
oxygen is: 6.04 milliliters (mL) of oxygen per liter of water. Nitrogen gas does dissolve in water.
2. Pure or Non-Polar compound dissolve in non-polar solvents.
3. Non-polar solvents are solvents that are not water or not water-like. Example: Benzene, Toluene, Hexane, Pentane or Kerosene. So wax will
dissolve in toluene and not in water. The nail-polish will not dissolve in water but will dissolve in the nail polish remover, namely, acetone.
4. Daily Formative Assessment:
Discuss why carbon dioxide behaves like a pure
covalent molecule although it is a polar molecule?
Provide neat diagrams to support your discussion.
With Neat diagrams, explain the Polar
Covalent Bond Formation in Water Molecule.
Explain what you understand by the term,
“dipoles”
Discuss why carbon dioxide is able to dissolve in
water to form drinking soda, which is chemically
carbonic acid.
Taking real-world examples, prove that oxygen is
dissolved in water.
Analogy: Homeopathy System of Medicine: similia similibus
curantur: Like cures the like
