
Essential Fish Habitat Assessment Technical Report
Technical Report
Essential Fish Habitat Assessment
Revolution Wind Offshore Wind Farm
Prepared for:
Revolution Wind, LLC
56 Exchange Terrace, Suite 300
Providence, RI 02903
Prepared by:
INSPIRE Environmental
513 Broadway, Suite 314
Newport, Rhode Island 02840
October 2020

Essential Fish Habitat Assessment Technical Report
i
Table of Contents
1.0 INTRODUCTION .................................................................................................................................................... 1
1.1 DESCRIPTION OF THE PROPOSED ACTION ............................................................................................................................... 1
1.2 REGULATORY CONTEXT AND RESOURCE DEFINITION ................................................................................................................ 2
1.3 REGULATORY COORDINATION AND REQUIRED PERMITS ............................................................................................................ 2
1.4 CONTENTS OF THIS TECHNICAL REPORT ................................................................................................................................. 3
2.0 AFFECTED ENVIRONMENT .................................................................................................................................... 4
2.1 METHODOLOGY ................................................................................................................................................................ 4
2.2 BASELINE CONDITIONS ....................................................................................................................................................... 4
2.2.1 Offshore ............................................................................................................................................................... 4
2.2.2 Coastal ................................................................................................................................................................. 5
2.2.3 Essential Fish Habitat Designations ..................................................................................................................... 6
2.2.4 Habitat Areas of Particular Concern .................................................................................................................... 8
2.2.5 Essential Fish Habitat Species and Life Stages ..................................................................................................... 9
2.3 SUMMARY OF EFH IN THE PROJECT AREA ............................................................................................................................ 33
3.0 ENVIRONMENTAL CONSEQUENCES AND PROTECTION MEASURES ...................................................................... 39
3.1 IMPACT ASSESSMENT....................................................................................................................................................... 39
3.1.1 Revolution Wind Farm ....................................................................................................................................... 39
3.1.2 Revolution Wind Export Cable ............................................................................................................................ 52
3.2 SUMMARY OF IMPACTS .................................................................................................................................................... 60
3.2.1 Summary of Impacts on EFH from RWF IPFs ...................................................................................................... 60
3.2.2 Summary of Impacts on EFH from RWEC IPFs .................................................................................................... 62
3.3 PROPOSED ENVIRONMENTAL PROTECTION MEASURES ........................................................................................................... 65
4.0 CONCLUSIONS .................................................................................................................................................... 66
5.0 REFERENCES ....................................................................................................................................................... 67

Essential Fish Habitat Assessment Technical Report
ii
Figures
FIGURE 1.1-1 MAP OF THE PROJECT AREA, INCLUDING THE POTENTIAL EXPORT CABLE CORRIDOR AND REVOLUTION WIND FARM. ....................... 1
FIGURE 2.2-1 TIDALLY-INFLUENCED HABITATS WITHIN THE PROJECT AREA .................................................................................................. 6
Tables
TABLE 2.2-1 EFH DESIGNATIONS FOR SPECIES IN THE RWF AND RWEC .................................................................................................... 7
TABLE 2.3-1 HABITAT PREFERENCES OF EARLY BENTHIC LIFE STAGES WITH EFH IN THE PROJECT AREA .......................................................... 34
TABLE 2.3-2 HABITAT PREFERENCES OF LATE BENTHIC LIFE STAGES WITH EFH IN THE PROJECT AREA ............................................................ 34
TABLE 2.3-3 EARLY PELAGIC LIFE STAGES WITH EFH IN THE PROJECT AREA ............................................................................................... 36
TABLE 2.3-4 LATE PELAGIC LIFE STAGES WITH EFH IN THE PROJECT AREA ................................................................................................. 37
TABLE 3.1-1 IPFS AND IMPACT CHARACTERIZATION FOR EFH WITHIN THE RWF DURING CONSTRUCTION AND DECOMMISSIONING ..................... 40
TABLE 3.1-2 IPFS AND IMPACT CHARACTERIZATION FOR EFH WITHIN THE RWF DURING OPERATIONS AND MAINTENANCE ............................... 47
TABLE 3.1-3 IPFS AND IMPACT CHARACTERIZATION FOR EFH FOR THE RWEC DURING CONSTRUCTION AND DECOMMISSIONING ....................... 53
TABLE 3.1-4 IPFS AND IMPACT CHARACTERIZATION FOR EFH FOR THE RWEC DURING OPERATIONS AND MAINTENANCE.................................. 58
TABLE 3.2-1 EFH SPECIES LEAST LIKELY TO EXPERIENCE IMPACTS – RWF ................................................................................................. 60
TABLE 3.2-2 EFH SPECIES MOST LIKELY TO EXPERIENCE NEGATIVE IMPACTS – RWF .................................................................................. 61
TABLE 3.2-3 EFH SPECIES THAT MAY EXPERIENCE BENEFICIAL EFFECTS – RWF ........................................................................................ 62
TABLE 3.2-4 EFH SPECIES LEAST LIKELY TO EXPERIENCE IMPACTS – RWEC............................................................................................... 63
TABLE 3.2-5 EFH SPECIES MOST LIKELY TO EXPERIENCE NEGATIVE IMPACTS – RWEC ................................................................................ 64
TABLE 3.2-6 EFH SPECIES THAT MAY EXPERIENCE BENEFICIAL EFFECTS – RWEC ...................................................................................... 65

Essential Fish Habitat Assessment Technical Report
iii
List of Acronyms
ASMFC Atlantic States Marine Fisheries Commission
CMECS Coastal and Marine Ecological Classification Standard
EEZ exclusive economic zone
EFH essential fish habitat
ESA Endangered Species Act
HAPC Habitat Area of Particular Concern
ICCAT International Commission for the Conservation of Atlantic Tunas
IPF impact-producing factor
Lease Commercial Lease of Submerged Lands for Renewable Energy Development on the Outer
Continental Shelf OCS-A 0486
Lease Area BOEM-designated Renewable Energy Lease Area OCS-A 0486
MHW mean high water
MSFCMA Magnuson-Stevens Fishery Conservation and Management Act
NEFMC New England Fishery Management Council
NEFSC Northeast Fisheries Science Center
NEPA National Environmental Policy Act
NOAA Fisheries National Marine Fisheries Service
O&M operations and maintenance
OCS Outer Continental Shelf
OnSS onshore substation
OSS offshore substation
Project Revolution Wind Farm Project
Project Area Proposed Wind Farm Area, Export Cable Corridor, and all onshore project facility locations
including the Onshore Transmission Cable Corridor, and Onshore Substation
RIGIS Rhode Island Geographic Information System
RWEC Revolution Wind Farm Export Cable
RWEC-RI Revolution Wind Farm Export Cable-Rhode Island State Waters
RWEC-OCS Revolution Wind Farm Export Cable-Outer Continental Shelf
RWF Revolution Wind Farm
TJB transition joint bay

Essential Fish Habitat Assessment Technical Report
iv
U.S.C. United States Code
WTG wind turbine generator

Essential Fish Habitat Assessment Technical Report
1
1.0 INTRODUCTION
1.1 Description of the Proposed Action
Revolution Wind, LLC (Revolution Wind), a 50/50 joint venture between Orsted North America Inc. (Orsted NA) and
Eversource Investment LLC (Eversource), proposes to construct and operate the Revolution Wind Farm Project
(hereinafter referred to as the Project). The wind farm portion of the Project will be located in federal waters on the
Outer Continental Shelf (OCS) in the designated Bureau of Ocean Energy Management (BOEM) Renewable
Energy Lease Area OCS-A 0486 (Lease Area). The Lease Area is approximately 20 statute miles (mi) (17.4 nautical
miles [nm], 30 kilometers [km]) south of the coast of Rhode Island (Figure 1.1-1).
Figure 1.1-1 Map of the Project Area, including the Potential Export Cable Corridor and Revolution Wind
Farm.
The Project will be comprised of both offshore and onshore components, which are described in detail in Section 3
of the Construction and Operations Plan.
Offshore:
• up to 100 Wind Turbine Generators (WTGs) connected by a network of Inter-Array Cables (IAC);
• up to two Offshore Substations (OSSs) connected by an OSS-Link Cable; and

Essential Fish Habitat Assessment Technical Report
2
• up to two submarine export cables (referred to as the RWEC), generally co-located within a single
corridor.
Onshore:
• a landfall location located at Quonset Point in North Kingstown, Rhode Island (referred to as the Landfall
Work Area);
• up to two underground transmission circuits (referred to as the Onshore Transmission Cable), co-
located within a single corridor; and
• a new Onshore Substation (OnSS) located adjacent to the existing Davisville Substation with up to two
interconnection circuits (overhead or underground) connecting the OnSS to the existing substation.
The Project’s components are further grouped into four general categories: the Revolution Wind Farm (RWF),
inclusive of the WTGs, OSSs, IAC, and OSS-Link Cable; the RWEC–OCS, inclusive of up to 25 mi (40 km) of the
RWEC in federal waters; the RWEC–RI State Waters, inclusive of up to 23 mi (37 km) of the RWEC in state waters;
and Onshore Facilities, inclusive of an up to 328-foot (ft) (100-meter [m]) segment of the RWEC, Landfall Work
Area, Onshore Transmission Cable, and OnSS (including interconnection circuits). Power from the RWF will be
delivered to the electric grid via two distinct transmission cable segments: the RWEC and the Onshore Transmission
Cable. The intersect of the RWEC and Onshore Transmission Cable will occur at co-located transition joint bays
(TJBs), which will be located at the Landfall Work Area. Multiple landfall sites are currently being evaluated within
the Landfall Work Area.
The Project will be commissioned and operational by end of Q4 2023. Revolution Wind assumes all permits will be
obtained in Q3 2022. It is further assumed construction will begin by the end of Q3 2022 with installation of the
onshore components and initiation of seabed preparation activities (clearing of debris and obstructions).
1.2 Regulatory Context and Resource Definition
Coastal and marine natural resources in the United States are governed and managed by multiple entities at the
federal, state, interstate, and tribal level. The Magnuson-Stevens Fishery Conservation and Management Act
(MSFCMA), passed in 1976, established eight regional fishery management councils for the conservation and
management of fisheries from 3 to 200 miles (4.8 to 322 km, 2.6 to 133.8 nm) off the U.S. coast. Fisheries and
stocks within 3 nm (5.6 km) of shore are managed by state governments. In the greater Atlantic region, management
of certain fisheries that are shared coastal resources is coordinated through the Atlantic States Marine Fisheries
Commission (ASMFC). The MSFCMA was revised and amended in 1996 with the passage of the Sustainable
Fisheries Act to strengthen conservation and increase the focus on sustainability, in part by requiring the
identification of essential fish habitat (EFH) (16 United States Code [U.S.C.] 1801-1884). The MSFCMA was again
revised and reauthorized in 2007, with additional conservation and management requirements to further the effort
against overfishing, support conservation, and improve fisheries science research (16 U.S.C. 1801-1884).
The MSFCMA was established, along with other goals, to promote the protection of EFH in the review of projects
conducted under federal permits, licenses, or other authorities that affect or have the potential to affect such habitat.
EFH is defined in the MSFCMA as those waters (e.g., aquatic areas and their associated physical, chemical, and
biological properties used by fish) and substrate (e.g., sediment, hard bottom, underlying structures, and associated
biological communities) necessary for the spawning, feeding, or growth to maturity of managed fish species.
Managed species include marine, estuarine, and anadromous finfish; mollusks; and crustaceans.
1.3 Regulatory Coordination and Required Permits
Federal agencies that authorize, fund, or undertake activities that may adversely affect EFH must consult with the
National Oceanic Atmospheric Administration’s National Marine Fisheries Service (NOAA Fisheries). An adverse
effect includes direct or indirect physical, chemical, or biological alterations, including changes to waters or
substrate, species and their habitat, other ecosystem components, or the quality and/or quantity of EFH. Although

Essential Fish Habitat Assessment Technical Report
3
absolute criteria have not been established for conducting EFH consultations, the guidelines issued by NOAA
Fisheries recommend consolidated EFH consultations with interagency coordination procedures required by other
statutes, such as the National Environmental Policy Act ( NEPA) or the Endangered Species Act (ESA), to reduce
duplication and improve efficiency. Generally, the EFH consultation process includes the following steps:
1. Notification – The action agency provides notification of the action to NOAA Fisheries.
2. EFH Assessment – The action agency prepares and submits an EFH Assessment that includes both
identification of affected EFH and an assessment of effects. Required elements of the assessment include
a description of the proposed action; an analysis of the potential adverse effects of that action on EFH and
the managed species; the federal action agency’s conclusions regarding the effects of the action on EFH;
and proposed environmental protection measures, if applicable.
3. EFH Conservation Recommendations – After reviewing the EFH Assessment, NOAA Fisheries provides
recommendations to the action agency regarding measures that can be taken by that agency to conserve
EFH.
4. Agency Response – Within 30 days of receiving the recommendations, the action agency must respond to
NOAA Fisheries with information on how it will proceed with the action. The response must include a
description of measures proposed by the agency to avoid, mitigate, or offset the impact of the activity on
EFH. For any conservation recommendation that is not adopted, the action agency must explain its reason
to NOAA Fisheries for not following the recommendation.
This technical report was prepared to provide federal permitting authorities (e.g., BOEM, ACOE) with the information
necessary to complete EFH consultation with NOAA Fisheries, as well as to facilitate BOEM’s review of the Project
under NEPA.
1.4 Contents of This Technical Report
Section 2.0 of this technical report describes the species and life stages with designated EFH, as well as Habitat
Areas of Particular Concern (HAPCs), that may occur within the RWF area and/or the RWEC corridor. Potential
impacts and environmental protection measures are discussed in Section 3.0.

Essential Fish Habitat Assessment Technical Report
4
2.0 AFFECTED ENVIRONMENT
2.1 Methodology
EFH data and text descriptions were downloaded from the NOAA Habitat Conservation EFH Mapper, an online
mapping application (NOAA Fisheries, 2019a) and supplemented with additional literature sources where
necessary. EFH data were queried using GIS software based on RWF and RWEC Project components and
manually verified. A 0.5-mile buffer centered on the RWEC route was assumed in order to query the data.
2.2 Baseline Conditions
2.2.1 Offshore
The RI-MA WEA is located offshore on the northeastern Atlantic continental shelf in Rhode Island Sound. The
waters in the vicinity of the RWF and RWEC are transitional waters that separate Narragansett Bay and Long Island
Sound from the outer continental shelf (OCS). Organisms that inhabit in these areas are adapted to survive in this
dynamic environment. In general, the benthic communities of these OCS areas are diverse, with lower densities of
organisms than in the northern portion of the Mid-Atlantic Bight and in deeper areas of the OCS (MMS, 2007). The
RI-MA WEA is composed of a mix of soft and hard bottom environments defined by dominant sediment grain size
and composition. Due to light requirements, SAV beds are limited to shallower depths and thus, do not occur within
the RI-MA WEA. However, SAV beds are found in parts of Narragansett Bay, Rhode Island, through which the
RWEC-RI transits before making landfall.
Based on data from site-specific benthic habitat surveys conducted for the Project (Benthic Assessment; INSPIRE
Environmental, 2020), across the vast majority of the RWEC-OCS and the northern region of the RWF, the
predominant habitat type was sand sheet, aside from a cluster of 4 stations in the northern center of the RWF that
consisted of a variety of habitat types including patchy pebbles on sand with mobile gravel, patchy cobbles and
boulders on sand, and sand with mobile gravel. Other regions of the RWF such as the southwest region of the RWF
and the central and southern portions of the RWF, tended to have more heterogeneous habitat types composed of
patchy pebbles on sand with mobile gravel, patchy cobbles on sand, and patchy boulders on sand. As a result of
the more heterogeneous physical composition and generally coarser substrates, these benthic environments
harbored more diverse epifaunal assemblages compared to the northern region of the RWF and the RWEC-OCS
stations.
In general, stations sampled along the RWEC-RI were low in environmental complexity, consisting mainly of sand
sheet habitat type. The exception was stations located in central Narragansett Bay, which were characterized by
the Coastal and Marine Ecological Classification Standard (CMECS) Biotic Subclass Attached Fauna and included
the habitat types of mollusk bed (or shells) on mud and patchy cobbles on sand. Along the RWEC-RI there were
spatial trends associated with the observed biological and physical features. The up-estuary stations were generally
characterized by finer substrate, dominated by soft-sediment fauna, higher turbidity, and more reduced sediments.
The mid-bay stations were characterized by mussel and Crepidula beds with other attached organisms including
barnacles, sponges, and macroalgae. The stations at the mouth of Narragansett Bay and the stations leading
offshore to the 3-mile state water boundary were generally dominated by soft sediment infauna.
Benthic communities have experienced increased water temperatures in the Project Area in the past several
decades, and average pH is expected to continue to decline as seawater becomes more saturated with carbon
dioxide (Saba et al., 2016). Acidification of seawater is associated with decreased survival and health of organisms
with calcareous shells (such as the Atlantic scallop, blue clam, and hard clam), but less is known about direct effects
of acidification on cartilaginous and bony fishes.
Modeled scenarios of decreasing seawater pH predict a substantial decline in the harvestable stock of the Atlantic
scallop, with collateral loss of economic value (Rheuban et al., 2018). Numerous benthic and pelagic species are
predicted to shift their ranges northward and into deeper waters in response to increasing water temperatures

Essential Fish Habitat Assessment Technical Report
5
(Selden et al., 2018; Kleisner et al., 2017). The ranges of dozens of groundfish species in New England waters
have shifted northward and into deeper waters in response to increasing water temperatures (Pinsky et al., 2013;
Nye et al., 2009) and more species are predicted to follow (Selden et al., 2018; Kleisner et al., 2017). The black sea
bass, identified as particularly sensitive to habitat alteration (Guida et al., 2017), has been increasing in abundance
over the past several years, and is expected to continue its expansion in southern New England as water
temperatures increase (Kuffner, 2018; McBride et al., 2018). Several pelagic forage species have been increasing
in the Project Area, including butterfish, scup, squid (Collie et al., 2008) and Atlantic mackerel (McManus et al.,
2018). Perhaps counterintuitively, distributions of other species are reported to be shifting southward, including
spiny dogfish, little skate, and silver hake (Walsh et al., 2015). It has been suggested that the spiny dogfish may
replace the Atlantic cod as a major predator in southern New England as the cod is driven north by warm waters
that the spiny dogfish tolerates well (Selden et al., 2018). Further temperature increases in southern New England
are expected to exceed the global ocean average by at least a factor of two, and ocean circulation patterns are
projected to change (Saba et al., 2016). Distributional shifts are occurring in both demersal and pelagic species,
perhaps mediated by changes in spawning locations and dates (Walsh et al., 2015). Southern species, including
some highly migratory species such as mahi that prefer warmer waters, are expected to follow the warming trend
and become more abundant in the area (Walsh et al., 2015; South Atlantic Fishery Management Council, 2003).
Climate change may also be affecting the migrations of anadromous fish in the region. The herrings, shad, and
sturgeon were identified as having high biological sensitivity to adverse effects of climate change (Hare et al., 2016).
In addition to physiological effects of temperature and pH, anadromous fishes face a physical risk caused by
flooding in their spawning rivers.
Modeling predicts that bottom temperatures in southern New England will become too warm to support larval
development of the commercially valuable American lobster, causing this species to move offshore and northward
(Rheuban et al., 2017). Lobster catches have declined in recent decades, which may be attributable to increases
water temperatures and associated increases in shell disease (Groner et al., 2018; Jaini et al., 2018; Collie and
King, 2016; Wahle et al., 2015). Egg-bearing female lobsters occur in warm coastal water in spring but may
aggregate offshore for spawning where waters are cooler and strong currents are favorable for larval transport
(Carloni et al., 2018). Larval lobster may be transported from Georges Bank to Rhode Island waters by currents
along the continental shelf during the 2 to 9 weeks of development to recruitment size (Carloni et al., 2018).
2.2.2 Coastal
The RWEC will make landfall at Quonset Point in North Kingstown, Rhode Island, where multiple locations for the
Landfall Work Area are being evaluated. Given that multiple locations are under consideration, a Landfall Envelope
has been identified to characterize the range of baseline conditions that may be affected by the Landfall Work Area.
Coastal habitats within the Landfall Envelope and vicinity include coastal beach, coastal dune, and tidal salt marsh
habitats (Figure 2-2-1). These habitats were delineated, photographed, characterized, and mapped during 2019
and 2020 field surveys to identify baseline conditions (Onshore Natural Resources & Biological Assessment; VHB,
2020).

Essential Fish Habitat Assessment Technical Report
6
Figure 2.2-1 Tidally-influenced Habitats within the Project Area
Most of the coastal habitats in the area proximate to the Landfall Envelope are disturbed from previous
anthropogenic uses. At Blue Beach, the open beach habitat consists of sand and the dune vegetation is made up
of American beach grass (Ammophila breviligulata), seaside goldenrod (Solidago sempervirens), rough cocklebur
(Xanthium strumarium), prickly lettuce (Lactuca serriola), switch grass (Panicum virgatum), spotted knapweed
(Centaurea stoebe), orangegrass (Hypericum gentianoides), common evening-primrose (Oenothera biennis), and
spearscale orache (Atriplex patula). Non-native plant species were observed within the coastal beach and coastal
dune area, but none of these species are documented as invasive. The landward side of the coastal dune at Blue
Beach transitions to tidal salt marsh. This wetland is likely infrequently inundated during extremely high tides and
storm surge events. The central area of the marsh bordering Blue Beach is dominated by saltmeadow cordgrass
(Spartina patens) and the perimeter is mostly composed of common reed (Phragmites australis), maritime marsh-
elder (Iva frutescens) and groundsel tree (Bacharis halimifolia). The common reed that occurs along the perimeter
of the tidal salt marsh is considered invasive. A tidal channel (potentially manmade) flows through the length of the
saltmarsh and connects to the inland freshwater forested swamp near the Blue Beach access path from Circuit
Drive.
Th eastern reach of Blue Beach has been altered with a seawall and riprap revetment such that the sandy beach
is exposed only during low tides. Vegetation that occurs at the base of the seawall and along the top of the seawall
includes spotted knapweed, common milkweed (Asclepias syriaca), prickly lettuce, and American pokeweed
(Phytolacca americana). Spotted knapweed is a weedy invasive species that occurs along the top of the seawall.
2.2.3 Essential Fish Habitat Designations
Within the RWF area, 40 species of fish and invertebrates have designated EFH for various life stages (Table 2.2-
1). Within the 0.5-mile (800-m) corridor around the RWEC centerline, 39 species of fish and invertebrates have

Essential Fish Habitat Assessment Technical Report
7
designated EFH with the RWEC-OCS, and 32 species have designated EFH within the RWEC-RI. Full descriptions
of each of these species and life stages are provided in Section 2.2.5.
Table 2.2-1 EFH Designations for Species in the RWF and RWEC
Table 2.2-1
Species Life Stages within RWF
Life Stages within RWEC-
OCS
Life Stages within RWEC-
RI
New England Finfish
Atlantic cod (Gadus morhua) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile Adult
Atlantic herring (Clupea harengus) Larvae, Juvenile, Adult Larvae, Juvenile, Adult Larvae, Juvenile, Adult
Atlantic wolfish (Anarhichas lupus) Egg, Larvae, Juvenile, Adult - -
Haddock (Melanogrammus aeglefinus) Egg, Larvae, Juvenile Larvae, Juvenile -
Monkfish (Lophius americanus) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Egg, Larvae
Ocean pout (Zoarces americanus) Egg, Juvenile, Adult Egg, Juvenile, Adult Egg, Juvenile, Adult
Pollock (Pollachius virens) Egg, Larvae, Juvenile Egg, Larvae, Juvenile Juvenile
Red hake (Urophycis chuss) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult
Silver hake (Merluccius bilinearis) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Egg, Larvae, Adult
White hake (Urophycis tenuis) Larvae, Juvenile Larvae, Juvenile Juvenile
Windowpane flounder (Scophthalmus
aquosus)
Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult
Winter flounder (Pseudopleuronectes
americanus)
Larvae, Juvenile, Adult Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult
Witch flounder (Glyptocephalus
cynoglossus)
Egg, Larvae Egg, Larvae -
Yellowtail flounder (Limanda ferruginea) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Juvenile, Adult
Mid-Atlantic Finfish
Atlantic butterfish (Peprilus triacanthus) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult
Atlantic mackerel (Scomber scombrus) Egg, Larvae, Juvenile Egg, Larvae, Juvenile Egg, Larvae, Juvenile, Adult
Black sea bass (Centropristis striata) Juvenile, Adult Juvenile, Adult Juvenile, Adult
Bluefish (Pomatomus saltatrix) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Juvenile, Adult
Scup (Stenotomus chrysops) Juvenile, Adult Juvenile, Adult Egg, Larvae, Juvenile, Adult
Summer flounder (Paralichthys dentatus) Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Larvae, Juvenile, Adult
Invertebrates
Atlantic sea scallop (Placopecten
magellanicus)
Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult Egg, Larvae, Juvenile, Adult
Atlantic surfclam (Spisula solidissima) - Adult Juvenile, Adult
Longfin inshore squid (Doryteuthis pealeii) Egg, Juvenile, Adult Egg, Juvenile, Adult Egg, Juvenile, Adult
Northern shortfin squid (Illex illecebrosus) Adult - -
Ocean quahog (Arctica islandica) Juvenile, Adult Juvenile, Adult -
Highly Migratory Species
Albacore tuna (Thunnus alalunga) Juvenile, Adult Juvenile, Adult Juvenile
Bluefin tuna (Thunnus thynnus) Juvenile, Adult Juvenile, Adult Juvenile, Adult
Skipjack tuna (Katsuwonus pelamis) Juvenile, Adult Adult Adult
Yellowfin tuna (Thunnus albacares) Juvenile, Adult Juvenile, Adult Juvenile
Skates

Essential Fish Habitat Assessment Technical Report
8
Table 2.2-1
Species Life Stages within RWF
Life Stages within RWEC-
OCS
Life Stages within RWEC-
RI
Little skate (Leucoraja erinacea) Juvenile, Adult Juvenile, Adult Juvenile, Adult
Winter skate (Leucoraja ocellata) Juvenile, Adult Juvenile, Adult Juvenile, Adult
Sharks
Basking shark (Cetorhinus maximus) Neonate, Juvenile, Adult Neonate, Juvenile, Adult -
Blue shark (Prionace glauca) Neonate, Juvenile, Adult Neonate, Juvenile, Adult -
Common thresher shark (Alopias vulpinus) Neonate, Juvenile, Adult Neonate, Juvenile, Adult Neonate, Juvenile, Adult
Dusky shark (Carcharhinus obscurus) Neonate, Juvenile, Adult Neonate, Juvenile, Adult -
Sand tiger shark (Carcharias taurus) Neonate, Juvenile Neonate, Juvenile Neonate, Juvenile
Sandbar shark (Carcharhinus plumbeus) Juvenile, Adult Juvenile, Adult Juvenile, Adult
Shortfin mako shark (Isurus oxyrinchus) Neonate, Juvenile, Adult Neonate, Juvenile, Adult -
Smoothhound shark complex (Atlantic
stock) (Mustelus canis)
Neonate, Juvenile, Adult Neonate, Juvenile, Adult Neonate, Juvenile, Adult
Spiny dogfish (Squalus acanthias) Sub-adult male, Sub-adult
female, Adult male, Adult
female
Sub-adult male, Sub-adult
female, Adult male, Adult
female
Sub-adult female, Adult
male
White shark (Carcharodon carcharias) Neonate, Juvenile, Adult Neonate, Juvenile, Adult Neonate
2.2.4 Habitat Areas of Particular Concern
Within the areas designated as EFH for various species, particular areas termed Habitat Areas of Particular Concern
(HAPCs) are also identified. HAPCs are discrete subsets of EFH that provide extremely important ecological
functions or are especially vulnerable to degradation, but this designation does not confer any particular protections.
The RWEC-RI corridor crosses HAPC for juvenile Atlantic cod in Rhode Island state waters. The juvenile cod HAPC
is a subset of the area designated as juvenile cod EFH, and is defined as the inshore areas of the Gulf of Maine
and Southern New England between 0 to 66 feet (0 to 20 m), relative to mean high water, as shown in Map 245 of
the Final Omnibus EFH Amendment 2 (New England Fishery Management Council [NEFMC], 2017). This HAPC
contains structurally complex rocky-bottom habitat that provides juvenile cod with protection from predation and
supports a wide variety of prey items (NEFMC, 2017).
Maps for summer flounder HAPC are not available for the Project Area, but it is defined as all native species of
macroalgae, seagrasses, and freshwater and tidal macrophytes in any size bed, as well as loose aggregations,
within adult and juvenile summer flounder EFH. Juvenile and adult summer flounder EFH is present within the RWF
area, RWEC-OCS, and RWEC-RI, but summer flounder HAPC, if present, is most likely to occur within Narragansett
Bay and nearshore portions of the Project Area. The Project does not cross known areas of submerged aquatic
vegetation, but during the site-specific benthic habitat surveys, isolated patches of attached macroflora were
observed at four stations along the RWEC in Narraganset Bay. Based on GIS analysis of available eelgrass
mapping for Narragansett Bay (Rhode Island Geographic Information System [RIGIS], 2017), a small section of
eelgrass is present on the western side of Dutch Island, approximately 679 feet (207 m) from the proposed RWEC
cable centerline. The next closest area of mapped eelgrass is on the western side of Conanicut Island,
approximately 1,411 feet (430 m) from the RWEC cable centerline. See the Benthic Assessment (INSPIRE
Environmental, 2020) for a detailed description of benthic habitats in the Project Area.

Essential Fish Habitat Assessment Technical Report
9
2.2.5 Essential Fish Habitat Species and Life Stages
2.2.5.1 New England Finfish Species
2.2.5.1.1 Atlantic Cod
Atlantic cod have two separate stocks managed by NOAA Fisheries, the Gulf of Maine stock, and the Georges
Bank stock. Atlantic cod range from Greenland to Cape Hatteras, North Carolina, but are most common on Georges
Bank and in the western Gulf of Maine (NOAA Fisheries, 2019b). Atlantic cod can be found at depths between 32
and 492 feet (10 and 150 m), and spawn near the seafloor from winter to early spring (NOAA Fisheries, 2019b).
They are top predators in demersal habitats, and feed on a variety of invertebrates and fish. They prefer muddy,
gravelly, or rocky substrates. Atlantic cod are historically an important commercial and recreational species and are
still fished at low levels; however, as of the 2017 stock assessment, both stocks are considered overfished, and are
currently subject to overfishing (Northeast Fisheries Science Center [NEFSC], 2017a). Atlantic cod EFH
designations are listed below for the life stages found within the Project Area. Egg, larvae, juvenile, and adult life
stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Eggs: EFH is pelagic habitats in the Gulf of Maine, on Georges Bank, and in the Mid-Atlantic region, as shown on
Map 38 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and in the high salinity zones of the bays and
estuaries listed in Table 19 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017).
Larvae: EFH is pelagic habitats in the Gulf of Maine, on Georges Bank, and in the Mid-Atlantic region, as shown on
Map 39 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and in the high salinity zones of the bays and
estuaries listed in Table 19 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017).
Juveniles: EFH is intertidal and subtidal benthic habitats in the Gulf of Maine, southern New England, and on
Georges Bank, to a maximum depth of 394 feet (120 m) (see Map 40 in NEFMC [2017]), including high salinity
zones in the bays and estuaries listed in Table 19 of NEFMC (2017). Structurally complex habitats, including
eelgrass, mixed sand and gravel, and rocky habitats (gravel pavements, cobble, and boulder) with and without
attached macroalgae and emergent epifauna, are essential habitats for juvenile cod. In inshore waters, young-of-
the-year juveniles prefer gravel and cobble habitats and eelgrass beds after settlement, but in the absence of
predators also utilize adjacent unvegetated sandy habitats for feeding. Survival rates for young-of-the-year cod are
higher in more structured rocky habitats than in flat sand or eelgrass; growth rates are higher in eelgrass. Older
juveniles move into deeper water and are associated with gravel, cobble, and boulder habitats, particularly those
with attached organisms. Gravel is a preferred substrate for young-of-the-year juveniles on Georges Bank and they
have also been observed along the small boulders and cobble margins of rocky reefs in the Gulf of Maine.
Adults: EFH is subtidal benthic habitats in the Gulf of Maine, south of Cape Cod, and on Georges Bank, between
98 and 525 feet (30 and 160 m) (see Map 41 in NEFMC [2017]), including high salinity zones in the bays and
estuaries listed in Table 19 of NEFMC (2017). Structurally complex hard bottom habitats composed of gravel,
cobble, and boulder substrates with and without emergent epifauna and macroalgae are essential habitats for adult
cod. Adult cod are also found on sandy substrates and frequent deeper slopes of ledges along shore. South of
Cape Cod, spawning occurs in nearshore areas and on the continental shelf, usually in depths less than 230 feet
(70 m).
2.2.5.1.2 Atlantic Herring
Atlantic herring are managed in one stock complex encompassing Georges Bank and the Gulf of Maine, with two
major spawning components. Atlantic herring are a small schooling fish found on both sides of the North Atlantic.
In the western North Atlantic, Atlantic herring range from Labrador, Canada to Cape Hatteras, North Carolina
(NOAA Fisheries, 2019c) and are highly concentrated in Georges Bank, the Gulf of Maine, and Nantucket Shoals
(Reid et al., 1999). In the region of interest, Atlantic herring are typically present in the winter at average depths of
about 120 to 360 feet (36 to 110 m) (Collette and Klein-MacPhee, 2002). They feed on zooplankton, krill, and fish
larvae, and are an important species in the food web of the northwest Atlantic (NOAA Fisheries, 2019c). Spawning
grounds are limited to rocky, gravelly, or pebbly bottom and on clay, from 12 to 180 feet (3 to 55 m) deep (Collette

Essential Fish Habitat Assessment Technical Report
10
and Klein-MacPhee, 2002). Atlantic herring are an important commercial fishery in New England and their stock
biomass is currently well above target levels (NOAA Fisheries, 2019c). According to the 2018 stock assessment,
Atlantic herring are not overfished, and not currently subject to overfishing (NEFSC, 2018a).
The Atlantic herring EFH designations are reproduced below for the life stages found within the Project Area.
Larvae, juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Larvae: EFH is inshore and offshore pelagic habitats in the Gulf of Maine, on Georges Bank, and in the upper Mid-
Atlantic Bight, as shown on Map 99 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and in the bays and
estuaries listed in Table 30 of NEFMC (2017). Atlantic herring have a very long larval stage, lasting 4–8 months,
and are transported long distances to inshore and estuarine waters where they metamorphose into early stage
juveniles in the spring.
Juveniles: EFH is intertidal and subtidal pelagic habitats to 984 feet (300 m) throughout the region, as shown on
Map 100 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), including the bays and estuaries listed in Table
30 of NEFMC (2017). One and two-year old juveniles form large schools and make limited seasonal inshore-
offshore migrations. Older juveniles are usually found in water temperatures of 37 to 59 °F (3 to 15 °C) in the
northern part of their range and as high as 72 °F (22 °C) in the Mid-Atlantic. Young-of-the-year juveniles can tolerate
low salinities, but older juveniles avoid brackish water.
Adults: EFH is subtidal pelagic habitats with maximum depths of 984 feet (300 m) throughout the region, as shown
on Map 100 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), including the bays and estuaries listed in
Table 30 of NEFMC (2017). Adults make extensive seasonal migrations between summer and fall spawning
grounds on Georges Bank and the Gulf of Maine and overwintering areas in southern New England and the Mid-
Atlantic region. They seldom migrate beyond a depth of about 328 feet (100 m) and—unless they are preparing to
spawn—usually remain near the surface. They generally avoid water temperatures above 50 °F (10 °C) and low
salinities. Spawning takes place on the bottom, generally in depths of 41–194 feet (5–90 m) on a variety of
substrates.
2.2.5.1.3 Atlantic Wolffish
The Atlantic wolffish is found on both sides of the North Atlantic and infrequently in the Arctic. In the northwestern
Atlantic, they range from Davis Strait, Canada, to Cape Hatteras, North Carolina (Fisheries and Oceans Canada,
2018a). In U.S. waters, the species is managed as a single stock. Atlantic wolffish prefer colder water temperatures
and prey mainly on brittle stars, sea urchins, crabs, and shrimp (Fisheries and Oceans Canada, 2018a). Adult
Atlantic wolffish generally move inshore to spawn during the spring and summer, establishing nesting sites on
boulders and in rocky crevices, which are guarded by the males until the eggs hatch in late summer and early fall
(Fisheries and Oceans Canada, 2018a). According to the 2017 stock assessment, Atlantic wolffish are overfished
and not currently experiencing overfishing (NEFSC, 2017a).
The Atlantic wolffish EFH designations are reproduced below for the life stages found within the Project Area. Egg,
larvae, juvenile, and adult life stages have EFH within the RWF area.
Eggs: EFH is subtidal benthic habitats at depths less than 328 feet (100 m) within the geographic area shown on
Map 43 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017). Wolffish egg masses are hidden under rocks
and boulders in nests.
Larvae: EFH is pelagic and subtidal benthic habitats within the geographic area shown on Map 43 of NEFMC (2017).
Atlantic wolffish larvae remain near the bottom for up to six days after hatching, but gradually become more buoyant
as the yolk sac is absorbed.
Juveniles: EFH is subtidal benthic habitats at depths of 230 to 604 feet (70 to 184 m) within the geographic area
shown on Map 43 of NEFMC (2017). Juvenile Atlantic wolffish do not have strong substrate preferences.
Adults: EFH is subtidal benthic habitats at depths less than 568 feet (173 m) within the geographic area shown on
Map 43 of NEFMC (2017). Adult Atlantic wolffish have been observed spawning and guarding eggs in rocky habitats

Essential Fish Habitat Assessment Technical Report
11
in less than 98 feet (30 m) of water in the Gulf of St. Lawrence and Newfoundland and in deeper (164 to 328 feet
[50 to100 m]) boulder reef habitats in the Gulf of Maine. Egg masses have been collected on the Scotian Shelf in
depths of 328 to 426 feet (100 to 130 m), indicating that spawning is not restricted to coastal waters. Adults are
distributed over a wider variety of sand and gravel substrates once they leave rocky spawning habitats, but are not
caught over muddy bottom.
2.2.5.1.4 Haddock
In the western North Atlantic, haddock range from Newfoundland to Cape May, New Jersey, with the highest
abundance on Georges Bank and in the Gulf of Maine (NOAA Fisheries, 2019d). Haddock in U.S. waters are
managed as two stocks: the Gulf of Maine stock and the Georges Bank stock. Haddock are found at depths ranging
from 59 to 1,148 feet (15 to 350 m) and there is a very minimal seasonal difference between depths aside from a
slightly wider range of depths in the fall (Cargnelli et al., 1999a). Haddock prefer gravely, pebbly, clay, and sandy
substrates and avoid ledges and large rocks (Collette and Klein-MacPhee, 2002). They spawn on eastern Georges
Bank, to the east of Nantucket Shoals, and along the Maine coast between January and June (NOAA Fisheries,
2019d). Haddock prey items include mollusks, worms, crustaceans, sea stars, sea urchins, sand dollars, brittle
stars, fish eggs, and occasionally small fish such as herring (NOAA Fisheries, 2019d). Adults sometimes eat small
fish, especially herring. As of the 2017 stock assessment, the Georges Bank and Gulf of Maine stocks are not
overfished and are not subject to overfishing (NEFSC, 2017a).
The haddock EFH designations are reproduced below for the life stages found within the Project Area. Egg, larvae,
and juveniles have EFH within the RWF area, and larvae and juveniles have EFH within the RWEC-OCS corridor.
Eggs: EFH is pelagic habitats in coastal and offshore waters in the Gulf of Maine, southern New England, and on
Georges Bank, as shown on Map 44 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017).
Larvae: EFH is pelagic habitats in coastal and offshore waters in the Gulf of Maine, the Mid-Atlantic, and on Georges
Bank, as shown on Map 45 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017).
Juveniles: EFH is subtidal benthic habitats between 131 and 459 feet (40 and 140 m) in the Gulf of Maine, on
Georges Bank and in the Mid-Atlantic region, and as shallow as 66 feet (20 m) along the coast of Massachusetts,
New Hampshire, and Maine, as shown on Map 46 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017). Young-
of-the-year juveniles settle on sand and gravel on Georges Bank, but are found predominantly on gravel pavement
areas within a few months after settlement. As they grow, they disperse over a greater variety of substrate types on
the bank. Young-of-the-year haddock do not inhabit shallow, inshore habitats.
2.2.5.1.5 Monkfish
Monkfish are found in the northwest Atlantic Ocean from the Grand Banks and northern Gulf of St. Lawrence south
to Cape Hatteras, NC. In U.S. waters, the monkfish fishery is divided into two management areas, north and south
of Georges Bank. According to the 2013 stock assessment, monkfish are not overfished and are not subject to
overfishing in either management area (NEFSC, 2013). Monkfish can tolerate a wide range of temperatures and
depths, and migrate seasonally to spawn and feed (NOAA Fisheries, 2019e). Monkfish are present from summer
to fall from the tideline down to 2,160 feet (658 m) (Collette and Klein-MacPhee, 2002). Monkfish prefer hard sand,
pebbly bottom, gravel, and broken shells for their habitats (Collette and Klein-MacPhee, 2002). Monkfish spawn
from February to October, producing very large buoyant mucoidal egg “veils.” They are opportunistic feeders with
prey including a wide range of benthic and pelagic fish and invertebrate species along with sea birds, and diving
ducks. Monkfish ambush their prey through rapidly opening their mouth, creating a vacuum, and sucking the prey
into their needle-like, backward curving teeth (NOAA Fisheries, 2019e). They also have a small, dangling
appendage in the back of their mouth to attract small fish.
The monkfish EFH designations are reproduced below for the life stages found within the Project Area. Eggs, larvae,
juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor. In the RWEC-RI corridor,
only EFH for eggs and larvae is present.

Essential Fish Habitat Assessment Technical Report
12
Eggs and Larvae: EFH is pelagic habitats in inshore areas, and on the continental shelf and slope throughout the
Northeast region, as shown on Map 82 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017). Monkfish larvae
are more abundant in the Mid-Atlantic region and occur over a wide depth range, from the surf zone to depths of
3,281 to 4,921 feet (1,000 to 1,500 m) on the continental slope. Monkfish egg veils and larvae are most often
observed during the months from March to September.
Juveniles: EFH is subtidal benthic habitats in depths of 164 to 1,312 feet (50 to 400 m) in the Mid-Atlantic, between
66 and 1,312 feet (20 and 400 m) in the Gulf of Maine, and to a maximum depth of 3,281 feet (1,000 m) on the
continental slope, as shown on Map 83 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017). A variety of
habitats are essential for juvenile monkfish, including hard sand, pebbles, gravel, broken shells, and soft mud; they
also seek shelter among rocks with attached algae. Juveniles collected on mud bottom next to rock-ledge and
boulder fields in the western Gulf of Maine were in better condition than juveniles collected on isolated mud bottom,
indicating that feeding conditions in these edge habitats are better. Young-of-the-year juveniles have been collected
primarily on the central portion of the shelf in the Mid- Atlantic, but also in shallow nearshore waters off eastern
Long Island, up the Hudson Canyon shelf valley, and around the perimeter of Georges Bank. They have also been
collected as deep as 2,953 feet (900 m) on the continental slope.
Adults: EFH is subtidal benthic habitats in depths of 164 to 1,312 feet (50 to 400 m) in southern New England and
Georges Bank, between 66 and 1,312 feet (20 and 400 m) in the Gulf of Maine, and to a maximum depth of 3,281
feet (1,000 m) on the continental slope, as shown on Map 84 of the Final Omnibus EFH Amendment 2 (NEFMC,
2017). EFH for adult monkfish is composed of hard sand, pebbles, gravel, broken shells, and soft mud. They seem
to prefer soft sediments (fine sand and mud) over sand and gravel, and, like juveniles, utilize the edges of rocky
areas for feeding.
2.2.5.1.6 Ocean Pout
The ocean pout is currently managed in two stocks, northern and southern, and ranges from Labrador, Canada to
Virginia (Steimle et al., 1999a). This finfish is typically present in southern New England from late summer to winter.
According to the 2017 stock assessment, ocean pout is overfished and is not currently experiencing overfishing
(NEFSC, 2017a). Ocean pout are found in habitats that contain sandy mud, “sticky” sand, broken bottom, or pebbles
and gravel (Collette and Klein-MacPhee, 2002). Juveniles and adults feed by filtering sediment for prey items, which
include polychaetes, mollusks, crustaceans, and echinoderms (Steimle et al., 1999a). They spawn in protected
habitats, such as rock crevices and man-made artifacts, where they lay eggs and engage in nest-guarding behavior
(Steimle et al., 1999a).
The ocean pout EFH designations are reproduced below for the life stages found within the Project Area. Eggs,
juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Eggs: EFH is hard bottom habitats on Georges Bank, in the Gulf of Maine, and in the Mid-Atlantic Bight (see Map
48 in NEFMC [2017]), as well as the high salinity zones of the bays and estuaries listed in Table 20 of NEFMC
(2017). Eggs are laid in gelatinous masses, generally in sheltered nests, holes, or rocky crevices. EFH for ocean
pout eggs occurs in depths less than 328 feet (100 m) on rocky bottom habitats.
Juveniles: EFH is intertidal and subtidal benthic habitats in the Gulf of Maine and on the continental shelf north of
Cape May, New Jersey, on the southern portion of Georges Bank, and in the high salinity zones of a number of
bays and estuaries north of Cape Cod, extending to a maximum depth of 394 feet (120 m) (see Map 49 and Table
20 in NEFMC [2017]). EFH for juvenile ocean pout occurs on a wide variety of substrates, including shells, rocks,
algae, soft sediments, sand, and gravel.
Adults: EFH is subtidal benthic habitats between 66 and 459 feet (20 and 140 m) in the Gulf of Maine, on Georges
Bank, in coastal and continental shelf waters north of Cape May, New Jersey, and in the high salinity zones of a
number of bays and estuaries north of Cape Cod (see Map 50 and Table 20 in NEFMC, 2017). EFH for adult ocean
pout includes mud and sand, particularly in association with structure-forming habitat types; i.e., shells, gravel, or
boulders. In softer sediments, they burrow tail first and leave a depression on the sediment surface. Ocean pout

Essential Fish Habitat Assessment Technical Report
13
congregate in rocky areas prior to spawning and frequently occupy nesting holes under rocks or in crevices in
depths less than 328 feet (100 m).
2.2.5.1.7 Pollock
Pollock range throughout the northwestern Atlantic Ocean and are most commonly found on the western Scotian
Shelf and in the Gulf of Maine (NOAA Fisheries, 2019f). They spawn multiple times per season between November
through February over hard, stony, or rocky ocean bottoms in the Gulf of Maine and on Georges Bank. Smaller
pollock in inshore waters prey on small crustaceans and fish, and larger pollock prey predominantly on fish, but
their diet also includes euphausiids and mollusks (NOAA Fisheries, 2019f; Cargnelli et al., 1999b). Pollock are a
schooling species with a semi-pelagic lifestyle, and they can be found throughout the water column (Cargnelli et
al., 1999b). Pollock are managed as a single stock, and according to the 2017 stock assessment, they are not
overfished and are not currently subject to overfishing (NEFSC, 2017a).
The pollock EFH designations are reproduced below for the life stages found within the Project Area. Eggs, larvae,
and juvenile life stages have EFH within the RWF area and RWEC-OCS corridor. Within the RWEC-RI corridor,
EFH is only present for juveniles.
Eggs: EFH is pelagic inshore and offshore habitats in the Gulf of Maine, on Georges Bank, and in southern New
England, as shown on Map 51 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), including the bays and
estuaries listed in Table 21 of (NEFMC, 2017).
Larvae: EFH is pelagic inshore and offshore habitats in the Gulf of Maine, on Georges Bank, and in the Mid-Atlantic
region, as shown on Map 52 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), including the bays and
estuaries listed in Table 21 of (NEFMC, 2017).
Juveniles: EFH is inshore and offshore pelagic and benthic habitats from the intertidal zone to 591 feet (180 m) in
the Gulf of Maine, in Long Island Sound, and Narragansett Bay, between 131 and 591 feet (40 and 180 m) on
western Georges Bank and the Great South Channel (see Map 53 in NEFMC [2017]), and in mixed and full salinity
waters in a number of bays and estuaries north of Cape Cod (Table 21 in NEFMC [2017]). EFH for juvenile pollock
consists of rocky bottom habitats with attached macroalgae (rockweed and kelp) that provide refuge from predators.
Shallow water eelgrass beds are also essential habitats for young-of-the-year pollock in the Gulf of Maine. Older
juveniles move into deeper water into habitats also occupied by adults.
2.2.5.1.8 Red Hake
Red hake are managed as two stocks, the Gulf of Maine and Northern Georges Bank (northern) stock, and the
Southern Georges Bank and Mid-Atlantic (southern) stock (Steimle et al., 1999b; NOAA Fisheries, 2019g). Red
hake range from Newfoundland to North Carolina, but are most abundant from the western Gulf of Maine through
southern New England waters (NOAA Fisheries, 2019g). During warmer seasons, red hake are common at depths
greater than 328 feet (100 m), and during colder months, their depth range is from 90 to 1,214 feet (30 to 370 m)
(Steimle et al., 1999b). Red hake prey consists primarily of crustaceans and fish such as haddock, silver hake, sea
robins, sand lance, mackerel, and small red hake (NOAA Fisheries, 2019g). This groundfish species prefers deep
water environments with bottom habitat consisting of both soft and pebbly substrate. Spawning occurs uniformly
from Georges Bank to Nova Scotia and typically occurs nearshore as early as June and continues through fall
(Collette and Klein-MacPhee, 2002). According to the 2018 stock assessment, both the northern and southern
stocks are not considered overfished and are not currently subject to overfishing (Alade and Traver, 2018).
The red hake EFH designations are reproduced below for the life stages found within the Project Area. Egg, larvae,
juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Eggs and Larvae: EFH is pelagic habitats in the Gulf of Maine, on Georges Bank, and in the Mid- Atlantic, as shown
on Map 77 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and in the bays and estuaries listed in Table
27 of NEFMC (2017).

Essential Fish Habitat Assessment Technical Report
14
Juveniles: EFH is intertidal and subtidal benthic habitats throughout the region on mud and sand substrates, to a
maximum depth of 262 feet (80 m), as shown on Map 77 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017),
including the bays and estuaries listed in Table 27 of NEFMC (2017). Bottom habitats providing shelter are essential
for juvenile red hake, including mud substrates with biogenic depressions, substrates providing biogenic complexity
(e.g., eelgrass, macroalgae, shells, anemone and polychaete tubes), and artificial reefs. Newly settled juveniles
occur in depressions on the open seabed. Older juveniles are commonly associated with shelter or structure and
often found inside live bivalves.
Adults: EFH is benthic habitats in the Gulf of Maine and the outer continental shelf and slope in depths of 164 to
2,461 feet (50 to 750 m) (see Map 78 in NEFMC [2017]) and as shallow as 66 feet (20 m) in a number of inshore
estuaries and embayments (see Table 27 in NEFMC [2017]) as far south as Chesapeake Bay. Shell beds, soft
sediments (mud and sand), and artificial reefs provide essential habitats for adult red hake. They are usually found
in depressions in softer sediments or in shell beds and not on open sandy bottom. In the Gulf of Maine, they are
much less common on gravel or hard bottom, but they are reported to be abundant on hard bottoms in temperate
reef areas of Maryland and northern Virginia.
2.2.5.1.9 Silver Hake
Two stocks of silver hake are managed in U.S. waters, the Gulf of Maine and Northern Georges Bank (northern)
stock and the Southern Georges Bank and Mid-Atlantic (southern) stock, which includes southern silver hake and
offshore hake (NOAA Fisheries, 2019h). Silver hake are found from Cape Sable, Nova Scotia to Cape Hatteras,
North Carolina and are concentrated in deep basins in the Gulf of Maine and along the continental slope in winter
and spring. White hake are voracious nocturnal feeders, preying on fish, crustaceans and squid (NOAA Fisheries,
2019h; Lock and Packer, 2004). White hake spawn along the coast of the Gulf of Maine from Cape Cod to Grand
Manan Island, on southern and southeastern Georges Bank, and in southern New England to the south of Martha’s
Vineyard (NOAA Fisheries, 2019h). Peak spawning occurs from May to June in the southern area of their range,
and from July to August in the northern area of their range (NOAA Fisheries, 2019h). The 2018 stock assessment
concluded that the both the northern and southern stock are not overfished and are not currently subject to
overfishing (Alade and Traver, 2018).
The silver hake EFH designations are reproduced below for the life stages found within the Project Area. Eggs,
larvae, juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Within the RWEC-
RI corridor, EFH is designated for eggs, larvae, and adults.
Eggs and Larvae: EFH is pelagic habitats from the Gulf of Maine to Cape May, New Jersey, including Cape Cod
and Massachusetts Bays (see Map 74 and Table 26 in NEFMC [2017]).
Juveniles: EFH is pelagic and benthic habitats in the Gulf of Maine, including the coastal bays and estuaries listed
in Table 26 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and on the continental shelf as far south as
Cape May, New Jersey, at depths greater than 33 feet (10 m) in coastal waters in the Mid-Atlantic and between
131 and 1,312 feet (40 and 400 m) in the Gulf of Maine, on Georges Bank, and in the middle continental shelf in
the Mid-Atlantic, on sandy substrates (see Map 75 in NEFMC [2017]). Juvenile silver hake are found in association
with sand-waves, flat sand with amphipod tubes, and shells, and in biogenic depressions. Juveniles in the New
York Bight settle to the bottom at mid-shelf depths on muddy sand substrates and find refuge in amphipod tube
mats.
Adults: EFH is pelagic and benthic habitats at depths greater than 115 feet (35 m) in the Gulf of Maine and the
coastal bays and estuaries listed in Table 26 of NEFMC (2017), between 230 and 1,312 feet (70 and 400 m) on
Georges Bank and the outer continental shelf in the northern portion of the Mid-Atlantic Bight, and in some shallower
locations nearer the coast, on sandy substrates (see Map 76 of NEFMC [2017]). Adult silver hake are often found
in bottom depressions or in association with sand waves and shell fragments. They have also been observed at
high densities in mud habitats bordering deep boulder reefs, resting on boulder surfaces, and foraging over deep
boulder reefs in the southwestern Gulf of Maine. This species makes greater use of the water column (for feeding,
at night) than red or white hake.

Essential Fish Habitat Assessment Technical Report
15
2.2.5.1.10 White Hake
White hake range from the Gulf of St. Lawrence to the Mid-Atlantic Bight, and the population is divided into two
stocks: a Canadian stock primarily occurring in the Gulf of St. Lawrence and Scotian Shelf, and a U.S. stock primarily
occurring in the Gulf of Maine and on Georges Bank. Their range also includes estuaries along the continental shelf
to the submarine canyons of the upper continental slope, as well as the deep, muddy basins of the Gulf of Maine
(Chang et al., 1999a). Early juveniles are pelagic before settling to muddy and fine-grained sandy bottom or eelgrass
habitats. Older juveniles feed on polychaetes, shrimps, and other crustaceans. Adults are demersal, prefer fine
grained, muddy substrates, and feed predominantly on fish (Chang et al., 1999a). The timing and extent of spawning
in southern New England waters is not well defined, but is thought to occur in early spring in deep waters along the
continental slope (Chang et al., 1999a). The most recent stock assessment for the U.S. stock of white hake
concluded that the stock is not overfished and not currently subject to overfishing (NEFSC, 2017a).
The white hake EFH designations are reproduced below for the life stages found within the Project Area. Larvae
and juvenile life stages have EFH within the RWF area and RWEC-OCS corridor. Within the RWEC-RI corridor,
only EFH for juveniles is present.
Larvae: EFH is pelagic habitats in the Gulf of Maine, in southern New England, and on Georges Bank, as shown in
Map 56 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017). Early stage white hake larvae have been
collected on the continental slope, but cross the shelf-slope front and use nearshore habitats for juvenile nurseries.
Larger larvae and pelagic juveniles have been found only on the continental shelf.
Juveniles: EFH is intertidal and subtidal estuarine and marine habitats in the Gulf of Maine, on Georges Bank, and
in southern New England, including mixed and high salinity zones in a number of bays and estuaries north of Cape
Cod (see Table 22 in NEFMC [2017]), to a maximum depth of 984 feet (300 m) (see Map 57 in NEFMC [2017]).
Pelagic phase juveniles remain in the water column for about 2 months. In nearshore waters, EFH for benthic phase
juveniles occurs on fine-grained, sandy substrates in eelgrass, macroalgae, and un-vegetated habitats. In the Mid-
Atlantic, most juveniles settle to the bottom on the continental shelf, but some enter estuaries, especially those in
southern New England. Older young-of-the-year juveniles occupy the same habitat types as the recently-settled
juveniles but move into deeper water (>164 feet [50 m]).
2.2.5.1.11 Windowpane Flounder
The windowpane flounder range extends from the Gulf of St. Lawrence to Florida, but the species is most abundant
from Georges Bank to Chesapeake Bay (Chang et al., 1999b). Windowpane flounder is managed as two stocks:
the Gulf of Maine-Georges Bank (northern) stock and the Southern New England-Middle Atlantic Bight (southern)
stock. Windowpane flounder spawning is thought to begin in February or March in inshore waters, peaking in the
Mid-Atlantic Bight in May, and extending into Georges Bank during the summer (Chang et al., 1999b). Windowpane
flounder typically prefer sandy bottom habitats and range from just below the tide line to 150 feet (46 m) deep
(Collette and Klein-MacPhee, 2002). They feed on small crustaceans and various fish larvae, including hakes and
tomcod (Chang et al., 1999b). The 2017 stock assessments concluded that the northern stock of windowpane
flounder is overfished, but not currently experiencing overfishing, and the southern stock is not overfished and not
experiencing overfishing (NEFSC, 2017a).
The windowpane flounder EFH designations are reproduced below for the life stages found within the Project Area.
Egg, larvae, juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI
corridor.
Eggs and Larvae: EFH is pelagic habitats on the continental shelf from Georges Bank to Cape Hatteras and in
mixed and high salinity zones of coastal bays and estuaries throughout the region (see Map 59, Map 60, and Table
23 in NEFMC [2017]).
Juveniles: EFH is intertidal and subtidal benthic habitats in estuarine, coastal marine, and continental shelf waters
from the Gulf of Maine to northern Florida, as shown on Map 61 of the Final Omnibus EFH Amendment 2 (NEFMC,
2017), including mixed and high salinity zones in the bays and estuaries listed in Table 23 of NEFMC (2017). EFH

Essential Fish Habitat Assessment Technical Report
16
for juvenile windowpane flounder is found on mud and sand substrates and extends from the intertidal zone to a
maximum depth of 197 feet (60 m). Young-of-the-year juveniles prefer sand over mud.
Adults: EFH is intertidal and subtidal benthic habitats in estuarine, coastal marine, and continental shelf waters from
the Gulf of Maine to Cape Hatteras, as shown on Map 62 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017),
including mixed and high salinity zones in the bays and estuaries listed in Table 23 of NEFMC (2017). Essential
fish habitat for adult windowpane flounder is found on mud and sand substrates and extends from the intertidal
zone to a maximum depth of 230 feet (70 m).
2.2.5.1.12 Winter Flounder
Winter flounder is managed as three stocks: the Gulf of Maine stock, Georges Bank stock, and the Southern New
England/Mid-Atlantic stock (NOAA Fisheries, 2019i). Winter flounder range from the Gulf of St. Lawrence to North
Carolina, and are found in estuaries and on the continental shelf. Winter flounder prefer muddy, sandy, cobbled,
gravelly, or boulder substrate in mostly nearshore environments (Pereira et al., 1999). Winter flounder spawn over
sandy bottoms and algal mats in shallow nearshore habitats during the winter and spring (NFMS, 2019i). They are
opportunistic feeders, and prey items include polychaetes, amphipods, shrimp, clams, capelin eggs, and fish
(Pereira et al., 1999; NOAA Fisheries, 2019i). The 2017 stock assessment concluded that spawning stock biomass
of the Georges Bank stock has been increasing since 2005, and the stock is not overfished and not subject to
overfishing (NEFSC, 2017a). The Southern New England/Mid-Atlantic stock is overfished, but not currently
experiencing overfishing (NEFSC, 2017a). The results for the Gulf of Maine stock were highly uncertain. The
authors were unable to determine an abundance estimate for the Gulf of Maine stock, but concluded that it is not
currently subject to overfishing (NEFSC, 2017a).
The winter flounder EFH designations are reproduced below for the life stages found within the Project Area. Larvae,
juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Egg, larvae, juvenile, and
adult life stages have EFH within the RWEC-RI corridor.
Eggs: EFH is subtidal estuarine and coastal benthic habitats from mean low water to 16 feet (5 m) from Cape Cod
to Absecon Inlet (39° 22’ N), and as deep as 230 feet (70 m) on Georges Bank and in the Gulf of Maine (see Map
63 in NEFMC [2017]), including mixed and high salinity zones in the bays and estuaries listed in Table 24 of NEFMC
(2017). The eggs are adhesive and deposited in clusters on the bottom. Essential habitats for winter flounder eggs
include mud, muddy sand, sand, gravel, macroalgae, and submerged aquatic vegetation. Bottom habitats are
unsuitable if exposed to excessive sedimentation which can reduce hatching success.
Larvae: EFH is estuarine, coastal, and continental shelf water column habitats from the shoreline to a maximum
depth of 230 feet (70 m) from the Gulf of Maine to Absecon Inlet (39° 22’ N), and including Georges Bank, as shown
on Map 65 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), including mixed and high salinity zones in the
bays and estuaries listed in Table 24 of NEFMC (2017). Larvae hatch in nearshore waters and estuaries or are
transported shoreward from offshore spawning sites where they metamorphose and settle to the bottom as
juveniles. They are initially planktonic but become increasingly less buoyant and occupy the lower water column as
they get older.
Juveniles: EFH is estuarine, coastal, and continental shelf benthic habitats from the Gulf of Maine to Absecon Inlet
(39° 22’ N), and including Georges Bank, as shown on Map 64 of the Final Omnibus EFH Amendment 2 (NEFMC,
2017), and in mixed and high salinity zones in the bays and estuaries listed in Table 24 of NEFMC (2017). Essential
fish habitat for juvenile winter flounder extends from the intertidal zone (mean high water) to a maximum depth of
197 feet (60 m) and occurs on a variety of bottom types, such as mud, sand, rocky substrates with attached
macroalgae, tidal wetlands, and eelgrass. Young-of-the-year juveniles are found inshore on muddy and sandy
sediments in and adjacent to eelgrass and macroalgae, in bottom debris, and in marsh creeks. They tend to settle
to the bottom in soft-sediment depositional areas where currents concentrate late-stage larvae and disperse into
coarser-grained substrates as they get older.
Adults: EFH is estuarine, coastal, and continental shelf benthic habitats extending from the intertidal zone (mean
high water) to a maximum depth of 230 feet (70 m) from the Gulf of Maine to Absecon Inlet (39° 22’ N), and including

Essential Fish Habitat Assessment Technical Report
17
Georges Bank, as shown on Map 65 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and in mixed and
high salinity zones in the bays and estuaries listed in Table 24 of NEFMC (2017). EFH for adult winter flounder
occurs on muddy and sandy substrates, and on hard bottom on offshore banks. In inshore spawning areas, EFH
includes a variety of substrates where eggs are deposited on the bottom.
2.2.5.1.13 Witch Flounder
Witch flounder are managed as a single stock and in U.S. waters, range from the Gulf of Maine to Cape Hatteras,
North Carolina (Cargnelli et al., 1999c). Witch flounder spawn from April to November in the Gulf of Maine/Georges
Bank region, and from April to August in the Mid-Atlantic Bight, peaking in the summer in both regions (Cargnelli et
al., 1999c). Primary prey items include polychaetes, crustaceans, mollusks, and echinoderms. As of the 2017 stock
assessment, witch flounder is overfished, overfishing status is unknown, and the condition of the stock is poor
(NEFSC, 2017a).
The witch flounder EFH designations are reproduced below for the life stages found within the Project Area. EFH
for eggs and larvae is present within the RWF area and RWEC-OCS corridor.
Eggs and Larvae: EFH is pelagic habitats on the continental shelf throughout the Northeast region, as shown on
Map 66 and Map 67 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017).
2.2.5.1.14 Yellowtail Flounder
In U.S. waters, yellowtail flounder are managed as three stocks: the Gulf of Maine/Cape Cod stock, the Georges
Bank stock, and the Southern New England/Mid-Atlantic stock. Yellowtail flounder range from Newfoundland to
Chesapeake Bay (NOAA Fisheries, 2019j). These bottom-dwelling finfish prefer habitats with a mixture of sand and
mud (Collette and Klein-MacPhee, 2002; Johnson et al., 1999), and spawn during the spring and summer (NFMS,
2019j). Adult prey items consist mainly of benthic macrofauna such as crustaceans and worms (NOAA Fisheries,
2019j; Johnson et al., 1999). As of the 2017 stock assessment (NEFSC, 2017a), all three stocks are overfished,
currently subject to overfishing, and drastically below the biomass target level. (Johnson et al., 1999).
The yellowtail flounder EFH designations are reproduced below for the life stages found within the Project Area.
Egg, larvae, juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Juvenile and
adult life stages have EFH within the RWEC-RI corridor.
Eggs: EFH is coastal and continental shelf pelagic habitats in the Gulf of Maine, on Georges Bank, and in the Mid-
Atlantic region as far south as the upper Delmarva peninsula, as shown on Map 70 of the Final Omnibus EFH
Amendment 2 (NEFMC, 2017), including the high salinity zones of the bays and estuaries listed in Table 25 of
NEFMC (2017).
Larvae: EFH is coastal marine and continental shelf pelagic habitats in the Gulf of Maine, and from Georges Bank
to Cape Hatteras, as shown on Map 71 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), including the
high salinity zones of the bays and estuaries listed in Table 25 of NEFMC (2017).
Juveniles: EFH is subtidal benthic habitats in coastal waters in the Gulf of Maine and on the continental shelf on
Georges Bank and in the Mid-Atlantic as shown on Map 72 of the Final Omnibus EFH Amendment 2 (NEFMC,
2017), including the high salinity zones of the bays and estuaries listed in Table 25 of NEFMC (2017). EFH for
juvenile yellowtail flounder occurs on sand and muddy sand between 66 and 262 feet (20 and 80 m). In the Mid-
Atlantic, young-of-the-year juveniles settle to the bottom on the continental shelf, primarily at depths of 131 to 230
feet (40 to 70 m), on sandy substrates.
Adults: EFH is subtidal benthic habitats in coastal waters in the Gulf of Maine and on the continental shelf on
Georges Bank and in the Mid-Atlantic as shown on Map 73 of the Final Omnibus EFH Amendment 2 (NEFMC,
2017), including the high salinity zones of the bays and estuaries listed in Table 25 of NEFMC (2017). EFH for adult
yellowtail flounder occurs on sand and sand with mud, shell hash, gravel, and rocks at depths between 82 and 295
feet (25 and 90 m).

Essential Fish Habitat Assessment Technical Report
18
2.2.5.2 Mid-Atlantic Finfish Species
2.2.5.2.1 Atlantic Butterfish
The Atlantic butterfish is a semi-pelagic fish that tends to form loose schools and ranges from Newfoundland to
Florida (NOAA Fisheries, 2019k). They are most commonly found from the Gulf of Maine to Cape Hatteras, North
Carolina (Cross et al., 1999; NFMS, 2019k). Butterfish are managed as one stock in the northern region (New
England to Cape Hatteras) and two stocks south of Cape Hatteras. Butterfish are present in New England waters
from spring to fall and are found from the surface to 180 feet (54 m) deep in the summer, but as deep as 690 feet
(210 m) in the winter (Collette and Klein-MacPhee, 2002). Butterfish prefer sandy bottom environments rather than
rocky environments. Spawning occurs on the continental shelf and in nearshore areas and is very common in Long
Island Sound and the New York Bight (Cross et al., 1999). As of the 2018 stock assessment (Adams, 2018), Atlantic
butterfish are not overfished and not subject to overfishing.
The Atlantic butterfish EFH designations are reproduced below for the life stages found within the Project Area.
Egg, larvae, juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI
corridor.
Eggs: EFH is pelagic habitats in inshore estuaries and embayments from Massachusetts Bay to the south shore of
Long Island, New York, in Chesapeake Bay, and on the continental shelf and slope, primarily from Georges Bank
to Cape Hatteras, North Carolina. EFH for Atlantic butterfish eggs is generally found over bottom depths of 4,921
feet (1,500 m) or less where average temperatures in the upper 656 feet (200 m) of the water column are 43.7 to
70.7 °F (6.5 to 21.5 °C).
Larvae: EFH is pelagic habitats in inshore estuaries and embayments in Boston harbor, from the south shore of
Cape Cod to the Hudson River, and in Delaware and Chesapeake bays, and on the continental shelf from the Great
South Channel (western Georges Bank) to Cape Hatteras, North Carolina. EFH for Atlantic butterfish larvae is
generally found over bottom depths between 134 and 1148 feet (41 and 350 m) where average temperatures in the
upper 656 feet (200 m) of the water column are 47 to 71 °F (8.5 to 21.5 °C).
Juveniles: EFH is pelagic habitats in inshore estuaries and embayments from Massachusetts Bay to Pamlico
Sound, North Carolina, in inshore waters of the Gulf of Maine and the South Atlantic Bight, and on the inner
continental shelf and OCS from southern New England to South Carolina. EFH for juvenile Atlantic butterfish is
generally found over bottom depths between 32 and 918 feet (10 and 280 m) where bottom water temperatures are
between 43 and 80 °F (6.5 and 27 °C) and salinities are above 5 ppt. Juvenile butterfish feed mainly on planktonic
prey.
Adults: EFH is pelagic habitats in inshore estuaries and embayments from Massachusetts Bay to Pamlico Sound,
North Carolina, inshore waters of the Gulf of Maine and the South Atlantic Bight, on Georges Bank, on the inner
continental shelf south of Delaware Bay, and on the OCS from southern New England to South Carolina. EFH for
adult Atlantic butterfish is generally found over bottom depths between 32 and 820 feet (10 and 250 m) where
bottom water temperatures are between 40 and 81 °F (4.5 and 27.5 °C) and salinities are above 5 ppt. Spawning
probably does not occur at temperatures below 59 °F (15 °C). Adult butterfish feed mainly on planktonic prey,
including squids and fishes.
2.2.5.2.2 Atlantic Mackerel
In the northwestern Atlantic, Atlantic mackerel range from Labrador to North Carolina (NOAA Fisheries, 2019l).
They are a pelagic, schooling species and are managed as a single stock. Mackerel spawn off the coast (10 to 30
miles offshore) in deeper waters in two groups. The southern group primarily spawns in the Mid-Atlantic Bight from
April to May, and the northern group spawns in the Gulf of St. Lawrence in June and July (NOAA Fisheries, 2019l).
There is no known preferred breeding habitat (Collette and Klein-MacPhee, 2002). Atlantic mackerel prey on
crustaceans (e.g., copepods, krill, and shrimp), fish, and ascidians (sea squirts) (NOAA Fisheries, 2019l). Prior to
the 2018 stock assessment, the status of Atlantic mackerel was unknown (NOAA Fisheries, 2019l). The 2018 stock

Essential Fish Habitat Assessment Technical Report
19
assessment concluded that Atlantic mackerel are overfished, subject to overfishing, and have been overfished for
nearly a decade (NEFSC, 2018b).
The Atlantic mackerel EFH designations are reproduced below for the life stages found within the Project Area.
Egg, larvae, and juvenile life stages have EFH within the RWF area and RWEC-OCS corridor. Egg, larvae, juvenile,
and adult life stages have EFH within the RWEC-RI corridor.
Eggs: EFH is pelagic habitats in inshore estuaries and embayments from Great Bay, New Hampshire to the south
shore of Long Island, New York, inshore and offshore waters of the Gulf of Maine, and on the continental shelf from
Georges Bank to Cape Hatteras, North Carolina (mostly north of 38°N). EFH for Atlantic mackerel eggs is generally
found over bottom depths of 328 feet (100 m) or less with average water temperatures of 43 to 54 °F (6.5 to 12.5
°C) in the upper 59 feet (15 m) of the water column.
Larvae: EFH is pelagic habitats in inshore estuaries and embayments from Great Bay, New Hampshire to the south
shore of Long Island, New York, inshore waters of the Gulf of Maine, and on the continental shelf from Georges
Bank to Cape Hatteras, North Carolina (mostly north of 38°N). EFH for Atlantic mackerel larvae is generally found
over bottom depths between 68 and 328 feet (21 and 100 m) with average water temperatures of 42 to 52 °F (5.5
to 11.5 °C) in the upper 656 feet (200 m) of the water column.
Juveniles: EFH is pelagic habitats in inshore estuaries and embayments from Passamaquoddy Bay and Penobscot
Bay, Maine to the Hudson River, in the Gulf of Maine, and on the continental shelf from Georges Bank to Cape
Hatteras, North Carolina. EFH for juvenile Atlantic mackerel is generally found over bottom depths between 32 and
360 feet (10 and 110 m) and in water temperatures of 41 to 68 °F (5 to 20 °C). Juvenile Atlantic mackerel feed
primarily on small crustaceans, larval fish, and other pelagic organisms.
Adults: EFH is pelagic habitats in inshore estuaries and embayments from Passamaquoddy Bay, Maine to the
Hudson River, and on the continental shelf from Georges Bank to Cape Hatteras, North Carolina. EFH for adult
Atlantic mackerel is generally found over bottom depths less than 558 feet (170 m) and in water temperatures of 41
to 68 °F (5 to 20 °C). Spawning occurs at temperatures above 45 °F (7 °C), with a peak between 48 and 57 °F (9
and 14 °C). Adult Atlantic mackerel are opportunistic predators feeding primarily on a wider range and larger
individuals of pelagic crustaceans than juveniles, but also on fish and squid.
2.2.5.2.3 Black Sea Bass
The black sea bass is a demersal finfish species that range from Nova Scotia to Florida and is managed as two
stocks: Mid-Atlantic and South-Atlantic (NOAA Fisheries, 2019m). Black sea bass spend the summer in northern
inshore waters at depths of less than 120 feet (37 m) and spend the winter in southern offshore waters at depths of
240 to 540 feet (73 to 165 m) (ASMFC, 2019a). Black sea bass prefer structured habitats such as reefs, pilings,
jetties, shipwrecks, and lobster pots along the continental shelf (Steimle et al., 1999c; ASMFC, 2019a). Black sea
bass spawn in May along the North Carolina coast, then spawn from the middle of May until the end of June in New
Jersey, New York, and southern New England waters (Collette and Klein-MacPhee, 2002). Black sea bass consume
a variety of prey items, but prefer crabs, shrimp, worms, small fish, and clams (NOAA Fisheries, 2019m). The most
recent stock assessments for black sea bass concluded that both the Mid-Atlantic and South Atlantic stocks are not
overfished and not subject to overfishing (NEFSC, 2017b; Southeast Data Assessment and Review [SEDAR],
2018).
The black sea bass EFH designations are reproduced below for the life stages found within the Project Area.
Juvenile and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Juveniles: Offshore, EFH is the demersal waters over the continental shelf (from the coast out to the limits of the
exclusive economic zone [EEZ]), from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent
of all the ranked squares of the area where juvenile black sea bass are collected in the NEFSC trawl survey. Inshore,
EFH is the estuaries where black sea bass are identified as being common, abundant, or highly abundant in the
Estuarine Living Marine Resources (ELMR) database for the "mixing" and "seawater" salinity zones. Juveniles are
found in the estuaries in the summer and spring. Generally, juvenile black sea bass are found in waters warmer

Essential Fish Habitat Assessment Technical Report
20
than 43 °F (6 °C) with salinities greater than 18 ppt and coastal areas between Virginia and Massachusetts, but
winter offshore from New Jersey and south. Juvenile black sea bass are usually found in association with rough
bottom, shellfish and eelgrass beds, and man-made structures in sandy-shelly areas; offshore clam beds and shell
patches may also be used for over-wintering.
Adults: Offshore, EFH is the demersal waters over the continental shelf (from the coast out to the limits of the EEZ),
from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent of all the ranked 10-minute
squares of the area where adult black sea bass are collected in the NEFSC trawl survey. Inshore, EFH is the
estuaries where adult black sea bass were identified as being common, abundant, or highly abundant in the ELMR
database for the "mixing" and "seawater" salinity zones. Black sea bass are generally found in estuaries from May
through October. Wintering adults (November through April) are generally offshore, south of New York to North
Carolina. Temperatures above 43 °F (6 °C) seem to be the minimum requirements. Structured habitats (natural and
man-made), sand, and shell are usually the substrate preference.
2.2.5.2.4 Bluefish
Bluefish are a migratory species that is found throughout the world in most temperate coastal regions except the
eastern Pacific. In the U.S., they range from Maine to eastern Florida and are managed as a single stock (NOAA
Fisheries, 2019n). Bluefish generally school by size, concentrating between Maine and Cape Hatteras, North
Carolina in the summer, and offshore between Cape Hatteras and Florida in the winter (ASMFC, 2019b). Bluefish
spawn multiple times in spring and summer, with discrete groups spawning at different times (NOAA Fisheries,
2019n; ASMFC, 2019b). Bluefish are voracious, opportunistic predators, preying on squid and fish, particularly
menhaden and smaller fish such as silversides (NOAA Fisheries, 2019n; ASMFC, 2019b). Based on the most
recent stock assessment, bluefish are not overfished and not subject to overfishing (NEFSC, 2015).
The bluefish EFH designations are reproduced below for the life stages found within the Project Area. Egg, larvae,
juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Juvenile and adult life
stages have EFH within the RWEC-RI corridor.
Eggs: North of Cape Hatteras, EFH is pelagic waters found over the continental shelf (from the coast out to the
limits of the EEZ) at mid-shelf depths, from Montauk Point, New York south to Cape Hatteras in the highest 90
percent of the area where bluefish eggs were collected in the Marine Resources Monitoring, Assessment, and
Prediction (MARMAP) surveys. Bluefish eggs are generally not collected in estuarine waters and thus there is no
EFH designation inshore. Generally, bluefish eggs are collected between April through August in temperatures
greater than 64 °F (18 °C) and normal shelf salinities (>31 ppt).
Larvae: North of Cape Hatteras, EFH is pelagic waters found over the continental shelf (from the coast out to the
limits of the EEZ) most commonly above 59 feet (15 m), from Montauk Point, New York south to Cape Hatteras, in
the highest 90 percent of the area where bluefish larvae were collected during the MARMAP surveys. EFH also
includes the "slope sea" and Gulf Stream between latitudes 29° 00 N and 40° 00 N. Bluefish larvae are not generally
collected inshore so there is not EFH designation inshore for larvae. Generally, bluefish larvae are collected April
through September in temperatures greater than 64 °F (18 °C) in normal shelf salinities (>30 ppt).
Juveniles: North of Cape Hatteras, EFH is pelagic waters found over the continental shelf (from the coast out to the
limits of the EEZ) from Nantucket Island, Massachusetts south to Cape Hatteras, in the highest 90 percent of the
area where juvenile bluefish are collected in the NEFSC trawl survey. EFH also includes the "slope sea" and Gulf
Stream between latitudes 29° 00 N and 40° 00 N. Inshore, EFH is all major estuaries between Penobscot Bay,
Maine and St. Johns River, Florida. Generally juvenile bluefish occur in North Atlantic estuaries from June through
October, Mid-Atlantic estuaries from May through October, and South Atlantic estuaries March through December,
within the "mixing" and "seawater" zones. Distribution of juveniles by temperature, salinity, and depth over the
continental shelf is undescribed.
Adults: North of Cape Hatteras, EFH is the pelagic waters found over the continental shelf (from the coast out to
the limits of the EEZ), from Cape Cod Bay, Massachusetts south to Cape Hatteras, in the highest 90 percent of the
area where adult bluefish were collected in the NEFSC trawl survey. Inshore, EFH is all major estuaries between

Essential Fish Habitat Assessment Technical Report
21
Penobscot Bay, Maine and St. Johns River, Florida. Adult bluefish are found in North Atlantic estuaries from June
through October, Mid-Atlantic estuaries from April through October, and in South Atlantic estuaries from May
through January in the "mixing" and "seawater" zones. Bluefish adults are highly migratory, and distribution varies
seasonally and according to the size of the individuals comprising the schools. Bluefish are generally found in
normal shelf salinities (>25 ppt).
2.2.5.2.5 Scup
Scup are a migratory, schooling species found in the northwest Atlantic Ocean, primarily between Cape Cod,
Massachusetts, and Cape Hatteras, North Carolina (NOAA Fisheries, 2019o). Scup are currently managed as two
stocks, the Mid-Atlantic/New England stock, and the South Atlantic stock. Scup spend the winter in offshore waters
between southern New Jersey and Cape Hatteras, migrating to more northern and inshore waters when water
temperatures begin to rise in spring and summer (ASMFC, 2019c). Scup are known to congregate in nearshore
areas of New England from early April to December, at depths between 270 and 420 feet (82 to 128 m) (Collette
and Klein-MacPhee, 2002). Scup spawn over weedy or sandy areas in southern New England between
Massachusetts Bay and the New York Bight between May and August, with peak spawning activity taking place in
June (NOAA Fisheries, 2019o). Scup prefer smooth to rocky bottom habitats and usually form schools around such
bottoms, feeding on demersal invertebrates. The 2017 stock assessment for the Mid-Atlantic/New England stock
indicated that scup are not overfished and not currently subject to overfishing (NEFSC, 2017c). The population
status of the South Atlantic stock has not been assessed (NOAA Fisheries, 2019o).
The scup EFH designations are reproduced below for the life stages found within the Project Area. Juvenile and
adult life stages have EFH within the RWF area and RWEC-OCS corridor. Egg, larvae, juvenile, and adult life stages
have EFH within the RWEC-RI corridor.
Eggs: EFH is estuaries where scup eggs were identified as common, abundant, or highly abundant in the ELMR
database for the "mixing" and "seawater" salinity zones. In general, scup eggs are found from May through August
in southern New England to coastal Virginia, in waters between 55 and 73 °F (12 to 23 °C) and in salinities greater
than 15 ppt.
Larvae: EFH is estuaries where scup were identified as common, abundant, or highly abundant in the ELMR
database for the "mixing" and "seawater" salinity zones. In general, scup larvae are most abundant nearshore from
May through September, in waters between 55 and 73 °F (12 to 23 °C) and in salinities greater than 15 ppt.
Juveniles: Offshore, EFH is the demersal waters over the continental shelf (from the coast out to the limits of the
EEZ), from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent of all the ranked 10-minute
squares of the area where juvenile scup are collected in the NEFSC trawl survey. Inshore, EFH is the estuaries
where scup has been identified as common, abundant, or highly abundant in the ELMR database for the "mixing"
and "seawater" salinity zones. In general, juvenile scup are found during the summer and spring in estuaries and
bays between Virginia and Massachusetts, in association with various sands, mud, mussel, and eelgrass bed type
substrates and in water temperatures greater than 45 °F (7 °C) and salinities greater than 15 ppt.
Adults: Offshore, EFH is the demersal waters over the continental shelf (from the coast out to the limits of the EEZ),
from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent of all the ranked 10-minute
squares of the area where adult scup are collected in the NEFSC trawl survey. Inshore, EFH is the estuaries where
scup has been identified as common, abundant, or highly abundant in the ELMR database for the "mixing" and
"seawater" salinity zones. Generally, wintering adults (November through April) are usually offshore, south of New
York to North Carolina, in waters above 45 °F (7 °C).
2.2.5.2.6 Summer Flounder
Summer flounder are found in inshore and offshore waters from Nova Scotia to the east coast of Florida,
concentrating in the Mid-Atlantic region from Cape Cod, Massachusetts to Cape Fear, North Carolina (NOAA
Fisheries, 2019p; ASMFC, 2019d). Summer flounder are managed as a single stock. Summer flounder move
offshore in the fall to depths of 120 to 600 feet (37 to 183 m) to spawn (ASMFC, 2019d). Spawning peaks in October

Essential Fish Habitat Assessment Technical Report
22
and November, and larvae migrate to inshore coastal and estuarine nursey areas (NOAA Fisheries, 2019p; ASMFC,
2019d). Adult summer flounder prefer sandy habitats, but can be found in a variety of habitat with both mud and
sand substrates (Packer et al., 1999). Summer flounder are ambush predators, and prey opportunistically on fish
and invertebrates including sea worms, squid, shrimp, and other crustaceans (ASFMC, 2019d). The 2019 stock
assessment concluded that summer flounder are not overfished and not subject to overfishing (NEFSC, 2019).
The summer flounder EFH designations are reproduced below for the life stages found within the Project Area. Egg,
larvae, juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Larvae, juvenile,
and adult life stages have EFH within the RWEC-RI corridor.
Eggs: North of Cape Hatteras, EFH is the pelagic waters found over the continental shelf (from the coast out to the
limits of the EEZ), from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent of the all the
ranked 10-minute squares for the area where summer flounder eggs are collected in the MARMAP survey. In
general, summer flounder eggs are found between October and May, being most abundant between Cape Cod and
Cape Hatteras, with the heaviest concentrations within 9 miles (14.5 km, 7.8 nm) of shore off New Jersey and New
York. Eggs are most commonly collected at depths of 30 to 360 feet (9 to 110 m).
Larvae: North of Cape Hatteras, EFH is the pelagic waters found over the continental shelf (from the coast out to
the limits of the EEZ), from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent of all the
ranked 10-minute squares for the area where summer flounder larvae are collected in the MARMAP survey.
Inshore, EFH is all the estuaries where summer flounder were identified as being present (rare, common, abundant,
or highly abundant) in the ELMR database, in the "mixing" (defined in ELMR as 0.5 to 25.0 ppt) and "seawater"
(defined in ELMR as greater than 25 ppt) salinity zones. In general, summer flounder larvae are most abundant
nearshore (12 to 50 miles [19 to 80.5 km, 10.4 to 43.4 nm] from shore) at depths between 30 to 230 feet (9 to 70
m). They are most frequently found in the northern part of the Mid-Atlantic Bight from September to February, and
in the southern part from November to May.
Juveniles: North of Cape Hatteras, EFH is the demersal waters over the continental shelf (from the coast out to the
limits of the EEZ), from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent of all the
ranked 10-minute squares for the area where juvenile summer flounder are collected in the NEFSC trawl survey.
Inshore, EFH is all the estuaries where summer flounder were identified as being present (rare, common, abundant,
or highly abundant) in the ELMR database for the "mixing" and "seawater" salinity zones. In general, juveniles use
several estuarine habitats as nursery areas, including salt marsh creeks, seagrass beds, mudflats, and open bay
areas in water temperatures greater than 37 °F (3 °C) and salinities from 10 to 30 ppt range.
Adults: North of Cape Hatteras, EFH is the demersal waters over the continental shelf (from the coast out to the
limits of the EEZ), from the Gulf of Maine to Cape Hatteras, North Carolina, in the highest 90 percent of all the
ranked 10-minute squares for the area where adult summer flounder are collected in the NEFSC trawl survey.
Inshore, EFH is the estuaries where summer flounder were identified as being common, abundant, or highly
abundant in the ELMR database for the "mixing" and "seawater" salinity zones. Generally, summer flounder inhabit
shallow coastal and estuarine waters during warmer months and move offshore on the outer continental shelf at
depths of 500 feet (152 m) in colder months.
2.2.5.3 Invertebrates
2.2.5.3.1 Atlantic Sea Scallop
The Atlantic sea scallop is managed as a single stock that ranges from Newfoundland to Cape Hatteras, North
Carolina (NOAA Fisheries, 2019q). Atlantic sea scallop occur along the continental shelf, typically at depths ranging
from 59 to 360 feet (18 to 110 m), and are generally found in seabed areas with coarse substrates consisting of
firm sand, gravel, shells, and rocks (Hart and Chute, 2004). The sea scallop spawning season is usually in the late
summer or early fall, and spawning may also occur in the spring in the Mid-Atlantic Bight (NOAA Fisheries, 2019q).
The 2018 stock assessment concluded that Atlantic sea scallop are not overfished and are not subject to overfishing
(NEFSC, 2018a).

Essential Fish Habitat Assessment Technical Report
23
The Atlantic sea scallop EFH designations are reproduced below for the life stages found within the Project Area.
Egg, larvae, juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI
corridor.
Eggs: EFH is benthic habitats in inshore areas and on the continental shelf as shown on Map 97 of the Final
Omnibus EFH Amendment 2 (NEFMC, 2017), in the vicinity of adult scallops. Eggs are heavier than seawater and
remain on the seafloor until they develop into the first free-swimming larval stage.
Larvae: EFH is benthic and water column habitats in inshore and offshore areas throughout the region, as shown
on Map 97 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017). Any hard surface can provide an essential
habitat for settling pelagic larvae (“spat”), including shells, pebbles, and gravel. They also attach to macroalgae and
other benthic organisms such as hydroids. Spat attached to sedentary branching organisms or any hard surface
have greater survival rates; spat that settle on shifting sand do not survive.
Juveniles: EFH is benthic habitats in the Gulf of Maine, on Georges Bank, and in the Mid-Atlantic, as shown on Map
97 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), in depths of 59 to 361 feet (18 to 110 m). Juveniles
(0.2 to 0.5 inch [5 to 12 mm] shell height) leave the original substrate on which they settle (see spat, above) and
attach themselves with byssal threads to shells, gravel, and small rocks (pebble, cobble), preferring gravel. As they
grow older, they lose their byssal attachment. Juvenile scallops are relatively active and swim to escape predation.
While swimming, they can be carried long distances by currents. Bottom currents stronger than 10 cm/sec retard
feeding and growth. In laboratory studies, maximum survival of juvenile scallops occurred between 34 and 59 °F
(1.2 and 15 °C) and above salinities of 25 ppt. On Georges Bank, age 1 juveniles are less dispersed than older
juveniles and adults and are mainly associated with gravel-pebble deposits. Essential habitats for older juvenile
scallops are the same as for the adults (gravel and sand).
Adults: EFH is benthic habitats in the Gulf of Maine, on Georges Bank, and in the Mid-Atlantic, as shown on Map
97 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017). Essential habitats for older juvenile and adult sea
scallops are found on sand and gravel substrates in depths of 59 to 361 feet (18 to 110 m), but they are also found
in shallower water and as deep as 591 feet (180 m) in the Gulf of Maine. In the Mid-Atlantic they are found primarily
between 148 and 246 feet (45 and 75 m) and on Georges Bank they are more abundant between 197 and 295 feet
(60 and 90 m). They often occur in aggregations called beds which may be sporadic or essentially permanent,
depending on how suitable the habitat conditions are (temperature, food availability, and substrate) and whether
oceanographic features (fronts, currents) keep larval stages in the vicinity of the spawning population. Bottom
currents stronger than 25 cm/sec inhibit feeding. Growth of adult scallops is optimal between 50 and 59 °F (10 and
15 °C) and they prefer full strength seawater.
2.2.5.3.2 Atlantic Surfclam
The Atlantic surfclam ranges from the southern Gulf of St. Lawrence to Cape Hatteras, North Carolina. The species
prefers sandy habitats along the continental shelf (Cargnelli et al., 1999d), and is most abundant on Georges Bank,
the south shore of Long Island, and along the coasts of New Jersey and the Delmarva Peninsula (NOAA Fisheries,
2019r). Atlantic surfclam spawn in the late spring through the early fall (NOAA Fisheries, 2019r). According to the
most recent stock assessment, Atlantic surfclam are not overfished and not subject to overfishing (NEFSC, 2016).
The Atlantic surfclam EFH designations are reproduced below for the life stages found within the Project Area.
Adults have EFH designated within the RWEC-OCS corridor, and juveniles and adults have EFH designated within
the RWEC-RI corridor.
Juveniles and Adults: EFH is throughout the substrate, to a depth of 3 feet (1 m) below the water/sediment interface,
within federal waters from the eastern edge of Georges Bank and the Gulf of Maine throughout the Atlantic EEZ, in
areas that encompass the top 90 percent of all the ranked 10-minute squares for the area where surfclams were
caught in the NEFSC surfclam and ocean quahog dredge surveys. Surfclams generally occur from the beach zone
to a depth of about 200 feet (656 m), but beyond about 125 feet (52 m) abundance is low.

Essential Fish Habitat Assessment Technical Report
24
2.2.5.3.3 Longfin Inshore Squid
The longfin squid is a pelagic, schooling species that ranges from Newfoundland to the Gulf of Venezuela. In U.S.
waters, longfin inshore squid are managed as a single stock and are most abundant between Georges Bank and
Cape Hatteras, North Carolina (NOAA Fisheries, 2019s). Longfin inshore squid have a very short life span (less
than 1 year), and spawn year-round with peak productions in winter and summer (NOAA Fisheries, 2019s). Juvenile
longfin inshore squid feed on plankton, and adults are aggressive hunters that feed on fish, crustaceans, and their
own species (NOAA Fisheries, 2019s). The 2017 stock assessment concluded that longfin inshore squid are not
overfished, but there was not enough information to determine whether the stock is experiencing overfishing
(Hendrickson, 2017).
The longfin inshore squid EFH designations are reproduced below for the life stages found within the Project Area.
Egg, juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Eggs: EFH for longfin inshore squid eggs occurs in inshore and offshore bottom habitats from Georges Bank
southward to Cape Hatteras. EFH for eggs is generally found where bottom water temperatures are between 50
and 73 °F (10 and 23 °C), salinities are between 30 and 32 ppt and depth is less than 164 feet (50 m). Longfin
inshore squid eggs have also been collected in bottom trawls in deeper water at various places on the continental
shelf. Like most loliginid squids, longfin inshore squid egg masses or “mops” are demersal and anchored to the
substrates on which they are laid, which include a variety of hard bottom types (e.g., shells, lobster pots, piers, fish
traps, boulders, and rocks), submerged aquatic vegetation (e.g., Fucus sp.), sand, and mud.
Juveniles (Pre-Recruits): EFH is pelagic habitats in inshore and offshore continental shelf waters from Georges
Bank to South Carolina, in the southwestern Gulf of Maine, and in embayments such as Narragansett Bay, Long
Island Sound, and Raritan Bay. EFH is generally found over bottom depths between 20 and 525 feet (6 and 160 m)
where bottom water temperatures are 47 to 76 °F (8.5 to 24.5 °C) and salinities are 28.5 to 36.5 ppt. Pre-recruits
migrate offshore in the fall where they overwinter in deeper waters along the edge of the shelf. They make daily
vertical migrations, moving in the water column at night and down in the daytime. Small immature individuals feed
on planktonic organisms while larger individuals feed on crustaceans and small fish.
Adults (Recruits): EFH is pelagic habitats in inshore and offshore continental shelf waters from Georges Bank to
South Carolina, in inshore waters of the Gulf of Maine, and in embayments such as Narragansett Bay, Long Island
Sound, Raritan Bay, and Delaware Bay. EFH is generally found over bottom depths between 20 and 656 feet (6
and 200 m) where bottom water temperatures are 47 to 57 °F (8.5 to 14 °C) and salinities are 24 to 36.5 ppt.
Recruits inhabit the continental shelf and upper continental slope to depths of 1,312 feet (400 m). They migrate
offshore in the fall and overwinter in warmer waters along the edge of the shelf. Like the pre-recruits, they make
daily vertical migrations. Individuals larger than 4.7 inches (12 cm) feed on fish and those larger than 6.3 inches (16
cm) feed on fish and squid. Females deposit eggs in gelatinous capsules which are attached in clusters to rocks,
boulders, and aquatic vegetation and on sand or mud bottom, generally in depths less than 164 feet (50 m).
2.2.5.3.4 Northern Shortfin Squid
The northern shortfin squid is a highly migratory species found in the northwest Atlantic Ocean between the
Labrador Sea and the Florida Straits (Hendrickson and Holmes, 2004). In U.S. waters, northern shortfin squid are
managed as a single stock. Northern shortfin squid have a very short life span (less than 1 year). The species
migrates onto the continental shelf in the spring, and migrates offshore in the late autumn, presumably to a winter
spawning site (Hendrickson and Holmes, 2004). Winter habitats of the species are not well known, and the only
confirmed spawning area is located in the Mid-Atlantic Bight at depths of 371 to 1,237 feet (113 to 377 m)
(Hendrickson and Holmes, 2004). It is unknown whether the stock of northern shortfin squid is overfished or
experiencing overfishing, as relative abundance and biomass indices are highly variable and lacking a trend (Mid-
Atlantic Fishery Management Council and NOAA Fisheries, 2018).
The northern shortfin squid EFH designation for adults is reproduced below; this is the only life stage with EFH
within the RWF area. Northern shortfin squid EFH is not found within the RWEC-OCS or RWEC-RI corridor.

Essential Fish Habitat Assessment Technical Report
25
Adults (Recruits): EFH is pelagic habitats on the continental shelf and slope from Georges Bank to South Carolina,
and in inshore and offshore waters of the Gulf of Maine. EFH for adult northern shortfin squid is generally found on
the shelf over bottom depths between 135 and 1,312 feet (41 and 400 m) where bottom temperatures are 40.1 to
58.1 °F (4.5 to 14.5 °C) and salinities are 34.5 to 36.5 ppt. They have also been caught in bottom trawls as deep
as 8,202 feet (2,500 m) in waters beyond the edge of the shelf and on Bear Seamount. Adults make daily vertical
migrations, moving up in the water column at night and down in the daytime. They feed primarily on fish and
euphausiids and are also cannibalistic (larger females consume smaller males).
2.2.5.3.5 Ocean Quahog
Ocean quahog are managed as a single stock and range from Newfoundland to Cape Hatteras. The highest
concentrations of ocean quahog are found are south of Nantucket to the Delmarva Peninsula in offshore waters
(Cargnelli et al., 1999e). The species prefers medium- to fine-grain sand, sandy mud, and silty sand (Cargnelli et
al., 1999e). Ocean quahogs spawn once a year in the summer or fall, but the spawning season can be extended
over several months (NOAA Fisheries, 2019t). The 2017 stock assessment concluded that ocean quahog are not
overfished and not subject to overfishing (NEFSC, 2017d).
The ocean quahog EFH designations are reproduced below for the life stages found within the Project Area.
Juvenile and adult life stages have EFH within the RWF area and RWEC-OCS corridor.
Juveniles and Adults: EFH is throughout the substrate, to a depth of 3 feet (1 m) below the water/sediment interface,
within federal waters from the eastern edge of Georges Bank and the Gulf of Maine throughout the Atlantic EEZ, in
areas that encompass the top 90 percent of all the ranked 10-minute squares for the area where ocean quahogs
were caught in the NEFSC surfclam and ocean quahog dredge surveys. Distribution in the western Atlantic ranges
in depths from 30 feet (9 m) to about 800 feet (244 m). Ocean quahogs are rarely found where bottom water
temperatures exceed 60 °F (16 °C) and occur progressively further offshore between Cape Cod and Cape Hatteras.
2.2.5.4 Highly Migratory Species
2.2.5.4.1 Albacore Tuna
Albacore Tuna is a circumglobal, epipelagic species that is managed in three stocks: North Atlantic, South Atlantic,
and Mediterranean (NOAA Fisheries, 2017). They travel in large schools that are sometimes mixed with other tuna
species (NOAA Fisheries, 2019u). Albacore tuna forage down to depth of 1,640 feet (500 m), preying
opportunistically on a wide variety of fishes and invertebrates (NOAA Fisheries, 2017). Albacore tuna spawn in the
spring and summer in the western tropical areas of the Atlantic, and then they move northward and use the central
and northern portions of the Atlantic as their wintering area (NOAA Fisheries, 2017). The most recent stock
assessment concluded that the North Atlantic stock of albacore tuna is not overfished, has rebuilt to target
population levels, and is not subject to overfishing (International Commission for the Conservation of Atlantic Tunas
[ICCAT], 2016a).
The albacore tuna EFH designations are reproduced below for the life stages found within the Project Area. Juvenile
and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Within the RWEC-RI corridor, only
juveniles have EFH.
Juveniles and Adults: EFH is offshore, pelagic habitats of the Atlantic Ocean from the outer edge of the U.S. EEZ
through Georges Bank to pelagic habitats south of Cape Cod, and from Cape Cod to Cape Hatteras, North Carolina.
EFH also includes offshore pelagic habitats near the outer U.S. EEZ between North Carolina and Florida, and
offshore pelagic habitats associated with the Blake Plateau. EFH also includes offshore pelagic habitats in the
western and central Gulf of Mexico.
2.2.5.4.2 Bluefin Tuna
Bluefin tuna are a highly migratory, epipelagic species managed in two stocks: western and eastern, separated by
the 45° W meridian (NOAA Fisheries, 2017). In the western Atlantic, bluefin tuna range from Newfoundland to the
Gulf of Mexico (NOAA Fisheries, 2019v). Bluefin tuna are thought to forage off the eastern U.S. and Canadian

Essential Fish Habitat Assessment Technical Report
26
coasts from June through March, migrating to spawning grounds in the Gulf of Mexico, Bahamas, and the Straits
of Florida in April and May, and then generally moving back to foraging grounds of the Gulf Stream and North
American continental shelf and slope waters, including the South and Mid-Atlantic Bight, the Gulf of Maine, and the
Nova Scotia Shelf (NOAA Fisheries, 2017). Adult bluefin tuna feed opportunistically on a variety of schooling fish,
cephalopods, and benthic invertebrates, including silver hake, Atlantic mackerel, Atlantic herring, krill, sandlance,
and squid (NOAA Fisheries, 2017). The 2017 stock assessment concluded that the western Atlantic bluefin tuna
stock is not subject to overfishing, but the information was insufficient to determine whether the stock status is
overfished (ICCAT, 2017; NOAA Fisheries, 2019v).
The bluefin tuna EFH designations are reproduced below for the life stages found within the Project Area. Juvenile
and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Juveniles: EFH is coastal and pelagic habitats of the Mid-Atlantic Bight and the Gulf of Maine, between southern
Maine and Cape Lookout, from shore (excluding Long Island Sound, Delaware Bay, Chesapeake Bay, and Pamlico
Sound) to the continental shelf break. EFH in coastal areas of Cape Cod are located between the Great South
Passage and shore. EFH follows the continental shelf from the outer extent of the U.S. EEZ on Georges Bank to
Cape Lookout. EFH is associated with certain environmental conditions in the Gulf of Maine (61 to 66 °F (16 to 19
°C); 0 to 131 feet (0 to 40 m) deep). EFH in other locations associated with temperatures ranging from 39 to 79 °F
(4 to 26 °C), often in depths of less than 66 feet (20 m) (but can be found in waters that are 131–328 feet (40–100
m) in depth in winter).
Adults: EFH is located in offshore and coastal regions of the Gulf of Maine the mid-coast of Maine to Massachusetts;
on Georges Bank; offshore pelagic habitats of southern New England; from southern New England to coastal areas
between the mouth of Chesapeake Bay and Onslow Bay, North Carolina; from coastal North Carolina south to the
outer extent of the U.S. EEZ, inclusive of pelagic habitats of the Blake Plateau, Charleston Bump, and Blake Ridge.
EFH also consists of pelagic waters of the central Gulf of Mexico from the continental shelf break to the seaward
extent of the U.S. EEZ between Apalachicola, Florida and Texas.
2.2.5.4.3 Skipjack Tuna
The skipjack tuna is a circumglobal, epipelagic species that is managed as two stocks, eastern and western.
Skipjack tuna in the western Atlantic range are found in tropical and warm-temperate waters from Newfoundland to
Brazil (NOAA Fisheries, 2017). They are a schooling species, and have been known to associate with birds, drifting
objects, whales, sharks, and other tunas (NOAA Fisheries, 2017). Skipjack tuna feed opportunistically on a variety
of fishes, cephalopods, crustaceans, mollusks, and sometimes other skipjack tuna (NOAA Fisheries, 2017; NOAA
Fisheries, 2019w). The species spawns throughout the year in warm equatorial waters and from spring to early fall
in subtropical waters (NOAA Fisheries, 2017). Based on the 2014 stock assessment, western Atlantic skipjack tuna
are not overfished and not subject to overfishing (ICCAT, 2014).
The skipjack tuna EFH designations are reproduced below for the life stages found within the Project Area. Juvenile
and adult life stages have EFH within the RWF area. Within the RWEC-OCS corridor and RWEC-RI corridor, only
adults have EFH.
Juveniles: EFH is offshore pelagic habitats seaward of the continental shelf break between the seaward extent of
the U.S. EEZ boundary on Georges Bank (off Massachusetts), coastal and offshore habitats between
Massachusetts and South Carolina, localized areas off Georgia and South Carolina, and from the Blake Plateau
through the Florida Straits. EFH also includes offshore waters in the central Gulf of Mexico from Texas through the
Florida Panhandle. In all areas, juveniles are found in waters greater than 66 feet (20 m).
Adults: EFH is coastal and offshore habitats between Massachusetts and Cape Lookout, North Carolina and
localized areas in the Atlantic off South Carolina and Georgia, and the northern east coast of Florida. EFH in the
Atlantic Ocean is also located on the Blake Plateau, in the Florida Straits through the Florida Keys, and areas in the
central Gulf of Mexico, offshore in pelagic habitats seaward of the southeastern edge of the West Florida Shelf to
Texas.

Essential Fish Habitat Assessment Technical Report
27
2.2.5.4.4 Yellowfin Tuna
The yellowfin tuna is a circumglobal, epipelagic species found in tropical and temperate waters (NOAA Fisheries,
2017). In the western Atlantic, yellowfin tuna are managed as a single stock and spawn from May to August in the
Gulf of Mexico and from July to November in the southeastern Caribbean (NOAA Fisheries, 2019x). The species
travel in schools, with juveniles found at the surface in mixed schools with other tuna species (NOAA Fisheries,
2017). Yellowfin tuna feed primarily in surface waters down to a depth of 328 feet (100 m), preying on a wide variety
of fish and invertebrates (NOAA Fisheries, 2017). According to the 2016 stock assessment, Atlantic yellowfin tuna
are not overfished and are not currently subject to overfishing (ICCAT, 2016b).
The yellowfin tuna EFH designations are reproduced below for the life stages found within the Project Area. Juvenile
and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Within the RWEC-RI corridor, only
juveniles have EFH.
Juveniles: EFH is offshore pelagic habitats seaward of the continental shelf break between the seaward extent of
the U.S. EEZ boundary on Georges Bank and Cape Cod, Massachusetts. EFH also includes offshore and coastal
habitats from Cape Cod to the mid-east coast of Florida and the Blake Plateau, locally distributed areas in the
Florida Straits and off the southwestern edge of the West Florida Shelf, the central Gulf of Mexico from the Florida
Panhandle to southern Texas, and localized areas southeast of Puerto Rico.
Adults: EFH is offshore pelagic habitats seaward of the continental shelf break between the seaward extent of the
U.S. EEZ boundary on Georges Bank and Cape Cod, Massachusetts. EFH also includes offshore and coastal
habitats from Cape Cod to North Carolina, offshore pelagic habitats of the Blake Plateau. EFH in the Gulf of Mexico
spans throughout much of the offshore pelagic habitat from the West Florida Shelf to the continental shelf off
southern Texas.
2.2.5.5 Skates
2.2.5.5.1 Little Skate
The little skate is a demersal species that ranges from Nova Scotia to Cape Hatteras and is most abundant in the
northern Mid-Atlantic Bight and on Georges Bank (Packer et al., 2003a). Little skate are managed as a single stock
as part of the Northeast Skate Complex
. The little skate is present in New England year-round, and mating may
take place at any time throughout the year, although there is evidence that most egg cases are found fully or partially
developed from late October to January and from June to July (Packer et al., 2003a). Little skate primarily prey on
decapod crustaceans, amphipods, and polychaetes, and to a lesser extent, isopods, bivalves, and fishes (Packer
et al., 2003a). According to the 2016 stock status update, little skate are not overfished and not experiencing
overfishing (Sosebee, 2017).
The little skate EFH designations are reproduced below for the life stages found within the Project Area. Juvenile
and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Juveniles: EFH is intertidal and subtidal benthic habitats in coastal waters of the Gulf of Maine and in the Mid-
Atlantic region as far south as Delaware Bay, and on Georges Bank, extending to a maximum depth of 262 feet (80
m), as shown on Map 90 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and including high salinity zones
in the bays and estuaries listed in Table 28 of NEFMC (2017). EFH for juvenile little skates occurs on sand and
gravel substrates, but they are also found on mud.
Adults: EFH is intertidal and subtidal benthic habitats in coastal waters of the Gulf of Maine and in the Mid-Atlantic
region as far south as Delaware Bay, and on Georges Bank, extending to a maximum depth of 328 feet (100 m),
as shown on Map 91 of the Final Omnibus EFH Amendment 2 (NEFMC, 2017), and including high salinity zones in
the bays and estuaries listed in Table 28 of NEFMC (2017). EFH for adult little skates occurs on sand and gravel
substrates, but they are also found on mud.

Essential Fish Habitat Assessment Technical Report
28
2.2.5.5.2 Winter Skate
Winter skate range from the Gulf of St. Lawrence in Canada to Cape Hatteras, North Carolina, and have
concentrated populations on Georges Bank and the northern section of the Mid-Atlantic Bight (Packer et al., 2003b;
NOAA Fisheries, 2019y). Winter skate are managed as a single stock as part of the Northeast Skate Complex
(NOAA Fisheries, 2019y). Mating is thought to take place year-round, though female winter skates with fully formed
egg capsules are more abundant in summer and fall (Packer et al., 2003b). Winter skate primarily prey on
polychaetes and amphipods, followed by decapod crustaceans, isopods, bivalves, and fishes (Packer et al., 2003b).
According to the most recent stock assessment, winter skate are not overfished and not experiencing overfishing
(Sosebee, 2017).
The winter skate EFH designations are reproduced below for the life stages found within the Project Area. Juvenile
and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Juveniles: EFH is subtidal benthic habitats in coastal waters from eastern Maine to Delaware Bay and on the
continental shelf in southern New England and the Mid-Atlantic region, and on Georges Bank, from the shoreline
to a maximum depth of 295 feet (90 m), as shown on Map 92 of the Final Omnibus EFH Amendment 2 (NEFMC,
2017), including the high salinity zones of the bays and estuaries listed in Table 28 of NEFMC (2017). EFH for
juvenile winter skates occurs on sand and gravel substrates, but they are also found on mud.
Adults: EFH is subtidal benthic habitats in coastal waters in the southwestern Gulf of Maine, in coastal and
continental shelf waters in southern New England and the Mid-Atlantic region, and on Georges Bank, from the
shoreline to a maximum depth of 262 feet (80 m), as shown on Map 93 of the Final Omnibus EFH Amendment 2
(NEFMC, 2017), including the high salinity zones of the bays and estuaries listed in Table 28 of NEFMC (2017).
EFH for adult winter skates occurs on sand and gravel substrates, but they are also found on mud.
2.2.5.6 Sharks
2.2.5.6.1 Basking Shark
The basking shark is a large, migratory species found in subpolar and cold temperate seas throughout the world
(NOAA Fisheries, 2017). In the western Atlantic, basking sharks are found in coastal regions from April to October,
with the highest abundance in May through August (NOAA Fisheries, 2017). Basking shark are filter-feeders that
feed swimming forward with an opened mouth to filter planktonic prey. Little is known about the reproductive habits
of basking shark, though aggregations of basking shark displaying courtship behaviors are thought to associate
with persistent thermal fronts in areas of high prey density (NOAA Fisheries, 2017). Harvest of basking shark is
prohibited in the U.S., and the species is listed as “Vulnerable” on the International Union for the Conservation of
Nature (IUCN) Red List of Threatened Species (Fowler, 2009). A stock assessment has not been conducted for
basking shark (NOAA Fisheries, 2017).
The basking shark EFH designations are reproduced below for the life stages found within the Project Area.
Neonate, juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor.
Neonate/Young-of-the-Year (YOY), Juveniles and Adults: At this time, insufficient data are available to differentiate
EFH between size classes; therefore, EFH designations for all life stages have been combined and are considered
the same. EFH is the Atlantic east coast from the Gulf of Maine to the northern Outer Banks of North Carolina, and
from mid-South Carolina to coastal areas of northeast Florida. Aggregations of basking sharks were observed from
the south and southeast of Long Island, east of Cape Cod, and along the coast of Maine, in the Gulf of Maine and
near the Great South Channel, approximately 59 miles (95 km) southeast of Cape Cod, Massachusetts as well as
approximately 47 miles (75 km) south of Martha’s Vineyard and 56 miles (90 km) south of Moriche’s Inlet, Long
Island. These aggregations tend to be associated with persistent thermal fronts within areas of high prey density.
2.2.5.6.2 Blue Shark
The blue shark is a common pelagic shark that ranges widely in tropical, subtropical, and temperate waters (NOAA
Fisheries, 2017). In the western Atlantic Ocean, they range from Newfoundland to Argentina (Fisheries and Oceans

Essential Fish Habitat Assessment Technical Report
29
Canada, 2018b). Blue shark migrate great distances and prefer deep, clear, blue waters, usually with temperatures
between 50 and 68 °F (10 and 20 °C) and depths greater than 591 feet (180 m) (NOAA Fisheries, 2017). Blue
sharks are thought to have an annual reproductive cycle, and nursery areas appear to be in open oceanic waters
in the higher latitudes of the its range (NOAA Fisheries, 2017). Blue shark prey mostly on squid and pelagic
schooling fishes, and are known to feed opportunistically on marine mammal and turtle carcasses (Fisheries and
Oceans Canada, 2018b). The 2015 stock assessment concluded that blue shark are not overfished and not
experiencing overfishing, though the authors acknowledged a high level of uncertainty in the results (ICCAT, 2015).
The blue shark EFH designations are reproduced below for the life stages found within the Project Area. Neonate,
juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor.
Neonates/YOY: EFH includes the Atlantic in areas offshore of Cape Cod through New Jersey, seaward of the 98
foot (30 m) bathymetric line (and excluding inshore waters such as Long Island Sound). EFH follows the continental
shelf south of Georges Bank to the outer extent of the U.S. EEZ in the Gulf of Maine.
Juveniles and Adults: EFH includes localized areas in the Atlantic Ocean in the Gulf of Maine, from Georges Bank
to North Carolina, South Carolina, Georgia, and off Florida.
2.2.5.6.3 Common Thresher Shark
The common thresher shark is a pelagic shark found in warm and temperate coastal and oceanic waters around
the world, with higher abundance near land (NOAA Fisheries, 2017). In the northwest Atlantic Ocean, they are
found from Newfoundland to Cuba. Common thresher shark prey on squid, pelagic crabs, and small fishes such as
anchovy, sardines, hakes, and small mackerels (NOAA Fisheries, 2017). Common thresher shark mating is thought
to occur in the late summer and fall, with females giving birth in spring (NOAA Fisheries, 2017; NOAA Fisheries,
2019z). A stock assessment has not been conducted for common thresher shark (NOAA Fisheries, 2019z).
The common thresher shark EFH designations are reproduced below for the life stages found within the Project
Area. Neonate, juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI
corridor.
Neonate/YOY, Juveniles, and Adults: At this time, insufficient data are available to differentiate EFH between the
juvenile and adult size classes; therefore, EFH is the same for those life stages. EFH is located in the Atlantic
Ocean, from Georges Bank (at the offshore extent of the U.S. EEZ boundary) to Cape Lookout, North Carolina; and
from Maine to locations offshore of Cape Ann, Massachusetts. EFH occurs with certain habitat associations in
nearshore waters of North Carolina, especially in areas with temperatures from 65 to 70 °F (18.2 to 20.9 °C) and at
depths from 15 to 45 feet (4.6 to 13.7 m).
2.2.5.6.4 Dusky Shark
The dusky shark is a migratory species found in warm and temperate waters over the continental shelf throughout
the Atlantic, Pacific, and Indian Oceans (NOAA Fisheries, 2017). The reproductive habits of dusky shark are not
well known, but the species is thought to give birth in Bulls Bay, South Carolina in April and May, and in the
Chesapeake Bay, Maryland in June and July (NOAA Fisheries, 2017). The shallow, coastal waters of
Massachusetts serve as nursery habitat for young dusky sharks. Dusky shark prey on a variety of fishes, squid,
and other elasmobranchs such as dogfish, catsharks, skates, and rays (Fisheries and Oceans Canada, 2018c;
Musick et al., 2009a). Harvest of dusky shark is prohibited in the U.S., and the species is listed as “Vulnerable” on
the IUCN Red List of Threatened Species (Musick et al., 2009a). The most recent stock assessment concluded that
dusky shark are overfished and subject to overfishing (SEDAR, 2016).
The dusky shark EFH designations are reproduced below for the life stages found within the Project Area. Neonate,
juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor.
Neonate/YOY: EFH in the Atlantic Ocean includes offshore areas of southern New England to Cape Lookout, North
Carolina. Specifically, EFH is associated with habitat conditions including temperatures from 65 to 72 °F (18.1 to

Essential Fish Habitat Assessment Technical Report
30
22.2 °C), salinities of 25 to 35 ppt and depths at 14 to 51 feet (4.3 to 15.5 m). The seaward extent of EFH for this
life stage in the Atlantic is 197 feet (60 m) in depth.
Juveniles and Adults: EFH is the coastal and pelagic waters inshore of the continental shelf break (< 656 feet [200
m] in depth) along the Atlantic east coast from habitats offshore of southern Cape Cod to Georgia, including the
Charleston Bump and adjacent pelagic habitats. The inshore extent for these life stages is the 66 foot (20 m)
bathymetric line, except in habitats of southern New England, where EFH is extended seaward of Martha’s
Vineyard, Block Island, and Long Island. EFH also includes pelagic habitats of southern Georges Bank and the
adjacent continental shelf break from Nantucket Shoals and the Great South Channel to the eastern boundary of
the United States EEZ. Adults are generally found deeper (to 6,562 feet [2,000 m]) than juveniles; however, there
is overlap in the habitats utilized by both life stages. In the Gulf of Mexico, EFH includes offshore waters of the
western and north Gulf, at and seaward of the continental shelf break, and in proximity to numerous banks along
the continental shelf edge (e.g., Ewing and Sackett Bank). The continental shelf edge habitat from Desoto Canyon
west to the Mexican border is important habitat for adult dusky sharks.
2.2.5.6.5 Sand Tiger Shark
Sand tiger shark are a large, coastal species found in tropical and warm temperate waters around the world, often
in very shallow water (13 feet [4 m]) (NOAA Fisheries, 2017). In the northwestern Atlantic, mature sand tiger shark
males and juveniles are found between Cape Cod and Cape Hatteras, and mature and pregnant females are found
between Cape Hatteras and Florida (NOAA Fisheries, 2017). Sand tiger reproductive habits are not well known,
but in the northwestern Atlantic they are thought to give birth in March and April. In the southern portions of its
range, females are believed to give birth in the winter, with neonates migrating northward to summer nurseries such
as Narragansett Bay (NOAA Fisheries, 2017). Sand tiger sharks feed on a variety of bony fishes, as well as other
elasmobranchs. Harvest of sand tiger shark is prohibited in the U.S., and the species is listed as “Vulnerable” on
the IUCN Red List of Threatened Species (Pollard and Smith, 2009).
The sand tiger shark EFH designations are reproduced below for the life stages found within the Project Area.
Neonate and juvenile life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Neonate/YOY and Juveniles: Neonate EFH ranges from Massachusetts to Florida, specifically the Plymouth,
Kingston, Duxbury Bay system, Sandy Hook, and Narragansett Bays as well as coastal sounds, lower Chesapeake
Bay, Delaware Bay (and adjacent coastal areas), Raleigh Bay and habitats surrounding Cape Hatteras. Juvenile
EFH includes habitats between Massachusetts and New York (Plymouth, Kingston, Duxbury Bay system), and
between mid-New Jersey and the mid-east coast of Florida. EFH can be described via known habitat associations
in the lower Chesapeake Bay and Delaware Bay (and adjacent coastal areas) where temperatures range from 66
to 77 °F (19 to 25 °C), salinities range from 23 to 30 ppt at depths of 9 to 23 feet (2.8 to 7.0 m) in sand and mud
areas, and in coastal North Carolina habitats with temperatures from 66 to 81 °F (19 to 27 °C), salinities from 30 to
31 ppt, depths of 27 to 45 feet (8.2 to 13.7 m), in rocky and mud substrate or in areas surrounding Cape Lookout
that contain benthic structure.
2.2.5.6.6 Sandbar Shark
The sandbar shark is a large, coastal species found in subtropical and warm temperate waters. In the northwestern
Atlantic, sandbar shark range from Cape Cod to the western Gulf of Mexico (NOAA Fisheries, 2017). Sandbar
sharks prefer bottom habitats and are most commonly found in 66 to 180 feet (20 to 55 m) of water, and occasionally
at depths of about 656 feet (200 m) (NOAA Fisheries, 2017). The species preys on a variety of bony fishes, other
elasmobranchs, mollusks, and crustaceans (Musick et al., 2009b). Sandbar sharks migrate seasonally, and males
and females segregate during most of the year (NFMS, 2017). Mating and birthing activities are thought to peak
between April and July, with most near-term pregnant and postpartum females observed in the Florida Keys (NOAA
Fisheries, 2017). In U.S. waters, sandbar shark nursery areas consist of shallow coastal waters from Cape
Canaveral, Florida to Martha’s Vineyard, Massachusetts. The 2017 stock assessment indicated that sandbar shark
are overfished and not experiencing overfishing (Southeast Data and Assessment Review, 2017).

Essential Fish Habitat Assessment Technical Report
31
The sandbar shark EFH designations are reproduced below for the life stages found within the Project Area.
Juvenile and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI corridor.
Juveniles: EFH includes coastal portions of the Atlantic Ocean between southern New England (Nantucket Sound,
Massachusetts) and Georgia in water temperatures ranging from 68 to 75 °F (20 to 24 °C) and depths from 7.9 to
21 feet (2.4 to 6.4 m). Important nurseries include Delaware Bay, Delaware, and New Jersey; Chesapeake Bay,
Virginia; Great Bay, New Jersey; and the waters off Cape Hatteras, North Carolina. For all EFH, water temperatures
range from 59 to 86 °F (15 to 30 °C), salinities range from 15 to 35 ppt, water depth ranges from 2.6 to 75 feet (0.8
to 23 m), and substrate includes sand, mud, shell, and rocky habitats. EFH in the Gulf of Mexico includes localized
areas off Apalachicola Bay, Florida.
Adults: EFH in the Atlantic Ocean includes coastal areas from southern New England to the Florida Keys, ranging
from inland waters of Delaware Bay and the mouth of Chesapeake Bay to the continental shelf break. EFH in the
Gulf of Mexico includes coastal areas between the Florida Keys and Anclote Key, Florida; areas offshore of the Big
Bend region; coastal areas of the Florida panhandle and Gulf coast between Apalachicola and the Mississippi River;
and habitats surrounding the continental shelf between Louisiana and south Texas. Adults commonly use habitats
in the West Florida Shelf, off Cape San Blas, and cool, deep, clear water offshore of Texas and Louisiana.
2.2.5.6.7 Shortfin Mako Shark
The shortfin mako shark is a highly migratory, pelagic species found in warm and warm-temperate waters around
the world. In eastern U.S. waters, shortfin mako shark are found from New England to Florida, in the Gulf of Mexico,
and in the Caribbean Sea. Shortfin mako prey on fast-moving fishes such as swordfish, tuna, and other sharks, as
well as other bony fishes, marine mammals, crustaceans, and cephalopods (NOAA Fisheries, 2017; NOAA
Fisheries, 2019aa). Shortfin mako reproductive habits and mating grounds are not well known, but mating is thought
to occur from summer to fall and pregnant females have only been captured between 20 and 30° N or S latitude
(NOAA Fisheries, 2017; NOAA Fisheries, 2019aa). According to the 2017 stock assessment, shortfin mako shark
are overfished and subject to overfishing (ICCAT, 2017).
The shortfin mako shark EFH designations are reproduced below for the life stages found within the Project Area.
Neonate, juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor.
Neonate/YOY, Juveniles, and Adults: At this time, available information is insufficient for the identification of EFH
by life stage, therefore all life stages are combined in the EFH designation. EFH in the Atlantic Ocean includes
pelagic habitats seaward of the continental shelf break between the seaward extent of the U.S. EEZ boundary on
Georges Bank (off Massachusetts) to Cape Cod (seaward of the 656-foot [200 m] bathymetric line); coastal and
offshore habitats between Cape Cod and Cape Lookout, North Carolina; and localized habitats off South Carolina
and Georgia. EFH in the Gulf of Mexico is seaward of the 656-foot (200 m) isobaths in the Gulf of Mexico, although
in some areas (e.g., northern Gulf of Mexico by the Mississippi delta) EFH extends closer to shore. EFH in the Gulf
of Mexico is located along the edge of the continental shelf off Fort Myers to Key West (southern West Florida
Shelf), and extends from the northern central Gulf of Mexico around Desoto Canyon and the Mississippi Delta to
pelagic habitats of the western Gulf of Mexico that are roughly in line with the Texas/Louisiana border.
2.2.5.6.8 Smoothhound Shark Complex (Atlantic Stock)
The smoothhound shark complex consists of three species: smooth dogfish (Mustelus canis), Florida smoothhound
(Mustelus norrisi), and Gulf smoothhound (Mustelus sinusmexicanus). Due to the difficulty in differentiating these
three species, EFH is designated for these sharks as a complex. However, smooth dogfish is the only smoothhound
shark complex species found in the Atlantic, so for the purposes of this report, we focus solely on smooth dogfish.
Smooth dogfish is a common, demersal coastal shark species that ranges from Massachusetts to northern
Argentina, typically inhabiting inshore waters down to 656 feet (200 m) (NOAA Fisheries, 2017). Smooth dogfish
migrate seasonally, congregating between the Chesapeake Bay and southern North Carolina in the winter, and
moving along the coast in the spring as waters warm (NOAA Fisheries, 2017). Smooth dogfish primarily consume
large crustaceans such as crabs and American lobster. During the spring in New England waters, smooth dogfish

Essential Fish Habitat Assessment Technical Report
32
are also known to feed on small bony fishes (NOAA Fisheries, 2017). Mating is through to occur between May and
September, and research suggests that estuaries are critically-important nursery habitats in the Mid-Atlantic Bight
(NOAA Fisheries, 2017). The 2015 stock assessment indicated that smooth dogfish are not overfished and not
experiencing overfishing (Southeast Data and Assessment Review, 2015).
The smoothhound shark complex EFH designations are reproduced below for the life stages found within the Project
Area. Neonate, juvenile, and adult life stages have EFH within the RWF area, RWEC-OCS corridor, and RWEC-RI
corridor.
Neonate/YOY, Juveniles, and Adults: At this time, available information is insufficient for the identification of EFH
for this life stage, therefore all life stages are combined in the EFH designation. Smoothhound shark EFH identified
in the Atlantic is exclusively for smooth dogfish. EFH in Atlantic coastal areas ranges from Cape Cod Bay,
Massachusetts to South Carolina, inclusive of inshore bays and estuaries (e.g., Pamlico Sound, Core Sound,
Delaware Bay, Long Island Sound, Narragansett Bay, etc.). EFH also includes continental shelf habitats between
southern New Jersey and Cape Hatteras, North Carolina.
2.2.5.6.9 Spiny Dogfish
The spiny dogfish is found in temperate and subarctic areas of the North Atlantic and North Pacific Oceans. In the
northwest Atlantic, their range extends from Labrador to Florida, which the highest concentrations between Nova
Scotia and Cape Hatteras, North Carolina (NOAA Fisheries, 2019ab). Spiny dogfish migrate seasonally, moving
north in the spring and summer and south in the fall and winter (ASMFC, 2019e). In Southern New England, spiny
dogfish abundance is highest in the fall (ASMFC, 2019e). Mating and birthing take place during the winter on
offshore wintering grounds (ASMFC, 2019e; NOAA Fisheries, 2019ab). Spiny dogfish are opportunistic feeders,
with smaller individuals primarily preying on crustaceans, and larger individuals preying on jellyfish, squid, and
schooling fishes (NOAA Fisheries, 2019ab). The 2018 stock assessment concluded that Atlantic spiny dogfish are
not overfished and not subject to overfishing (NOAA Fisheries, 2019ab).
The spiny dogfish EFH designations are reproduced below for the life stages found within the Project Area. Sub-
adult male, sub-adult female, adult male, and adult female life stages have EFH within the RWF area and RWEC-
OCS corridor. Sub-adult female and adult male life stages have EFH within the RWEC-RI corridor.
Sub-Adult Females: EFH is pelagic and epibenthic habitats throughout the region. Sub-adult females are found
over a wide depth range in full salinity seawater (32–35 ppt) where bottom temperatures range from 44.6 to 59 °F
(7 to 15 °C). Sub-adult females are widely distributed throughout the region in the winter and spring when water
temperatures are lower, but very few remain in the Mid-Atlantic area in the summer and fall after water temperatures
rise above 59 °F (15 °C).
Sub-Adults Males: EFH is pelagic and epibenthic habitats, primarily in the Gulf of Maine and on the outer continental
shelf from Georges Bank to Cape Hatteras. Sub-adult males are found over a wide depth range in full salinity
seawater (32–35 ppt) where bottom temperatures range from 44.6 to 59 °F (7 to 15 °C). Sub-adult males are not
as widely distributed over the continental shelf as the females and are generally found in deeper water. They are
widely distributed throughout the region in the winter and spring when water temperatures are lower, but very few
remain in the Mid-Atlantic area in the summer and fall after water temperatures rise above 59 °F (15 °C).
Adult Females: EFH is pelagic and epibenthic habitats throughout the region. Adults are found over a wide depth
range in full salinity seawater (32–35 ppt) where bottom temperatures range from 44.6 to 59 °F (7 to 15 °C). They
are widely distributed throughout the region in the winter and spring when water temperatures are lower, but very
few remain in the Mid-Atlantic area in the summer and fall after water temperatures rise above 59 °F (15 °C).
Adult Males: EFH is pelagic and epibenthic habitats throughout the region. Adults are found over a wide depth
range in full salinity seawater (32–35 ppt) where bottom temperatures range from 44.6 to 59 °F (7 to 15 °C). They
are widely distributed throughout the region in the winter and spring when water temperatures are lower, but very
few remain in the Mid-Atlantic area in the summer and fall after water temperatures rise above 59 °F (15 °C).

Essential Fish Habitat Assessment Technical Report
33
2.2.5.6.10 White Shark
The white shark is a large species found in coastal and offshore waters of cold and temperate seas (NOAA
Fisheries, 2017). In the northwestern Atlantic, white shark range sporadically from Newfoundland to the Gulf of
Mexico, but are most abundant on the continental shelf between Cape Hatteras and Cape Cod (NOAA Fisheries,
2017). White shark are seasonally common in some locations, including New England in the summer (NOAA
Fisheries, 2017). Juvenile white sharks prey primarily on fish, but shift to a diet of mostly marine mammals as they
grow (NOAA Fisheries, 2017). The reproductive habits of white sharks and locations of nursery areas are not well
known. Harvest of white shark is prohibited in the U.S., and the species is listed as “Vulnerable” on the IUCN Red
List of Threatened Species (Fergusson et al., 2009).
The white shark EFH designations are reproduced below for the life stages found within the Project Area. Neonate,
juvenile, and adult life stages have EFH within the RWF area and RWEC-OCS corridor. Within the RWEC-RI
corridor, only neonates have EFH.
Neonate/YOY: EFH includes inshore waters out to 65 miles (105 km) from Cape Cod, Massachusetts, to an area
offshore of Ocean City, New Jersey.
Juveniles and Adults: Known EFH includes inshore waters to habitats 65 miles (105 km) from shore, in water
temperatures ranging from 48 to 82 °F (9 to 28 °C), but more commonly found in water temperatures from 57 to 73
°F (14 to 23 °C) from Cape Ann, Massachusetts, including parts of the Gulf of Maine, to Long Island, New York,
and from Jacksonville to Cape Canaveral, Florida.
2.3 Summary of EFH in the Project Area
Tables 2.3-1 and 2.3-2 summarize early (i.e., eggs, larvae) and late (i.e., neonate, juveniles, adults) benthic life
stages of species with designated EFH in the Project Area, provide a description of preferred habitat, and provide
an assessment of whether the preferred habitat is present in the Project Area. Tables 2.3-3 and 2.3-4 summarize
the early and late pelagic life stages of species with designated EFH in the Project Area.

Essential Fish Habitat Assessment Technical Report
34
Table 2.3-1 Habitat Preferences of Early Benthic Life Stages with EFH in the Project Area
Table 2.3-1
Species Life Stage Location Description of Preferred Habitat
Preferred
Habitat Present
in Project Area?
Finfish
Atlantic wolffish Egg RWF Subtidal benthic habitats. Egg masses are hidden under
rocks and boulders in nests.
Yes
Larvae RWF Pelagic and subtidal benthic habitats. Yes
Ocean pout Egg RWF, RWEC-OCS,
RWEC-RI
Hard bottom habitats – sheltered nests, holes, and
crevices.
Limited
Winter flounder Egg RWEC-RI Bottom habitats with a substrate of mud, muddy sand,
sand, gravel, macroalgae, and submerged aquatic
vegetation.
Yes
Larvae RWF, RWEC-OCS,
RWEC-RI
Pelagic and bottom habitats. Yes
Invertebrates
Atlantic sea scallop Egg RWF, RWEC-OCS,
RWEC-RI
Coarse substrates of gravel, shells, and rocks. Yes
Larvae RWF, RWEC-OCS,
RWEC-RI
Hard surfaces for pelagic larvae to settle, including
shells, pebbles, and gravel. Larvae also attach to
macroalgae and other benthic organisms such as
hydroids.
Yes
Longfin inshore squid Egg RWF, RWEC-OCS,
RWEC-RI
Egg masses or “mops” are laid on a variety of
substrates, including hard bottom (shells, lobster pots,
fish traps, boulders, and rocks), submerged aquatic
vegetation (e.g. Fucus), sand, and mud.
Yes
Table 2.3-2 Habitat Preferences of Late Benthic Life Stages with EFH in the Project Area
Table 2.3-2
Species Life Stage Location Description of Preferred Habitat
Preferred
Habitat Present
in Project Area?
Finfish
Atlantic cod Juvenile RWF, RWEC-OCS,
RWEC-RI
Bottom habitats with a substrate of gravel or cobble, and
boulder habitats, especially those with attached
organisms.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Bottom habitats with a substrate of rocks, pebbles,
gravel, or boulders. Also found on sandy substrates.
Yes
Atlantic wolffish Juvenile RWF
Subtidal benthic habitats. Juveniles do not have strong
substrate preferences
Yes
Adult RWF
Subtidal benthic habitats, including a wide variety of
sand and gravel substrates. Rocky spawning habitats.
Yes
Black sea bass Juvenile RWF, RWEC-OCS,
RWEC-RI
Usually found in association with rough-bottom, shellfish
and eelgrass beds, and man-made structures in sandy-
shelly areas. Offshore clam beds and shell patches may
also be used during the winter.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Usually structured habitats (natural and man-made),
sand, and shell substrates.
Yes
Haddock Juvenile RWF, RWEC-OCS
Young-of-the-year juveniles settle on sand and gravel
but are found predominantly on gravel pavement areas.
Yes

Essential Fish Habitat Assessment Technical Report
35
Table 2.3-2
Species Life Stage Location Description of Preferred Habitat
Preferred
Habitat Present
in Project Area?
As they grow, they disperse over a greater variety of
substrate types.
Monkfish Juvenile
and Adult
RWF, RWEC-OCS
Bottom habitats with substrates of a sand-shell mix,
algae-covered rocks, hard sand, pebbly gravel, or soft
mud.
Yes
Ocean pout Juvenile RWF, RWEC-OCS,
RWEC-RI
Bottom habitats on a wide variety of substrates,
including shells, rocks, algae, soft sediments, sand, and
gravel.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Mud and sand, particularly in association with structure-
forming habitat types (i.e., shells, gravel, boulders).
Yes
Pollock Juvenile RWF, RWEC-OCS,
RWEC-RI
Rocky bottom habitats with attached macroalgae
(rockweed and kelp).
No
Red hake Juvenile RWF, RWEC-OCS,
RWEC-RI
Intertidal and subtidal benthic habitats on mud and sand
substrates. Bottom habitats providing shelter, including
mud substrates with biogenic depressions, substrates
providing biogenic complexity (e.g., eelgrass,
macroalgae, shells, anemone and polychaete tubes),
and artificial reefs. Newly settled juveniles occur in
depressions on the open seabed. Older juveniles are
commonly associated with shelter or structure and often
found inside live bivalves.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Shell beds, soft sediments (mud and sand), and artificial
reefs. Usually found in depressions in softer sediments
or in shell beds and not on open sandy bottom.
Yes
Scup Juvenile RWF, RWEC-OCS,
RWEC-RI
Associated with various sands, mud, mussel, and
eelgrass bed substrates
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Prefer smooth to rocky bottom habitats.
Yes
Silver hake Juvenile RWF, RWEC-OCS
Sandy substrates; found in association with sand waves,
flat sand with amphipod tubes, and shells, and in
biogenic depressions.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Pelagic and benthic habitats, including sandy
substrates, bottom depressions, mud habitats bordering
deep boulder reefs, boulder habitat, and associated with
sand waves and shell fragments.
Yes
Summer flounder Juvenile RWF, RWEC-OCS,
RWEC-RI
Prefer sandy or muddy bottom habitats. Use estuarine
habitats as nursery areas, including salt marsh creeks,
seagrass beds, mudflats, and open bay areas.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Prefer sandy or muddy bottom habitats. Inhabit shallow
coastal and estuarine waters.
Yes
White hake Juvenile RWF, RWEC-OCS,
RWEC-RI
Fine-grained, sandy substrates in eelgrass, macroalgae,
and unvegetated habitats.
Yes
Windowpane flounder Juvenile
and Adult
RWF, RWEC-OCS,
RWEC-RI
Bottom habitats with a substrate of mud or sand.
Yes
Winter flounder Juvenile RWF, RWEC-OCS,
RWEC-RI
Variety of bottom types such as mud, sand, rocky
substrates with attached macroalgae, tidal wetlands,
and eelgrass. Young-of-the-year juveniles are found
inshore on muddy and sandy sediments in and adjacent
to eelgrass and macroalgae, in bottom debris, and in
marsh creeks. They tend to settle to the bottom in soft-
sediment depositional areas and disperse into coarser-
grained substrates as they get older.
No

Essential Fish Habitat Assessment Technical Report
36
Table 2.3-2
Species Life Stage Location Description of Preferred Habitat
Preferred
Habitat Present
in Project Area?
Adult RWF, RWEC-OCS,
RWEC-RI
Muddy and sandy substrates, and on hard bottom on
offshore banks.
Yes
Yellowtail flounder Juvenile RWF, RWEC-OCS,
RWEC-RI
Sand and muddy sand.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Sand and sand with mud, shell hash, gravel, and rocks.
Yes
Invertebrates
Atlantic sea scallop Juvenile RWF, RWEC-OCS,
RWEC-RI
Bottom habitats with a substrate of shells, gravel, and
small rocks (pebble, cobble), preferring gravel.
Yes
Adult RWF, RWEC-OCS,
RWEC-RI
Bottom habitats with sand and gravel substrates.
Yes
Atlantic surfclam Juvenile RWEC-RI
Sandy habitats along the continental shelf.
Yes
Adult RWEC-OCS,
RWEC-RI
Sandy habitats along the continental shelf.
Yes
Ocean quahog Juvenile
and Adult
RWF, RWEC-OCS
Prefers medium to fine sandy bottom with mud and silt.
Yes
Skates
Little skate Juvenile
and Adult
RWF, RWEC-OCS,
RWEC-RI
Bottom habitats with a sandy or gravelly substrate, or
mud.
Yes
Winter skate Juvenile
and Adult
RWF, RWEC-OCS,
RWEC-RI
Bottom habitats with a substrate of sand and gravel or
mud.
Yes
Sharks
1
Spiny dogfish Sub-adult
male,
Adult
female
RWF, RWEC-OCS
Pelagic and epibenthic habitats.
Yes
Sub-adult
female,
Adult male
RWF, RWEC-OCS,
RWEC-RI
Pelagic and epibenthic habitats.
Yes
1
The neonate/young-of-the year life stage for shark species is more similar to a juvenile life stage than a larval life stage. Thus, neonate/young-
of-the year is considered to be a “late” life stage for the purpose of this analysis.
Table 2.3-3 Early Pelagic Life Stages with EFH in the Project Area
Table 2.3-3
Species Life Stage Location
Finfish
Atlantic butterfish Egg, Larvae RWF, RWEC-OCS, RWEC-RI
Atlantic cod Egg, Larvae RWF, RWEC-OCS, RWEC-RI
Atlantic herring Larvae RWF, RWEC-OCS, RWEC-RI
Atlantic mackerel Egg, Larvae RWF, RWEC-OCS, RWEC-RI
Atlantic wolffish Larvae RWF
Bluefish Egg, Larvae RWF, RWEC-OCS
Haddock Egg RWF

Essential Fish Habitat Assessment Technical Report
37
Table 2.3-3
Species Life Stage Location
Larvae RWF, RWEC-OCS
Monkfish Egg, Larvae RWF, RWEC-OCS, RWEC-RI
Pollock Egg, Larvae RWF, RWEC-OCS
Red hake Egg, Larvae RWF, RWEC-OCS, RWEC-RI
Scup Egg, Larvae RWEC-RI
Silver hake Egg, Larvae RWF, RWEC-OCS, RWEC-RI
Summer flounder Egg RWF, RWEC-OCS
Larvae RWF, RWEC-OCS, RWEC-RI
White hake Larvae RWF, RWEC-OCS
Windowpane flounder Egg, Larvae RWF, RWEC-OCS, RWEC-RI
Winter flounder Larvae RWF, RWEC-OCS, RWEC-RI
Witch flounder Egg, Larvae RWF, RWEC-OCS
Yellowtail flounder Egg, Larvae RWF, RWEC-OCS
Invertebrates
Atlantic sea scallop Larvae RWF, RWEC-OCS, RWEC-RI
Table 2.3-4 Late Pelagic Life Stages with EFH in the Project Area
Table 2.3-4
Species Life Stage Location
Finfish
Atlantic butterfish Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Atlantic herring Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Atlantic mackerel Juvenile RWF, RWEC-OCS, RWEC-RI
Adult RWEC-RI
Bluefish Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Pollock Juvenile RWF, RWEC-OCS, RWEC-RI
Silver hake Adult RWF, RWEC-OCS, RWEC-RI
White hake Juvenile RWF, RWEC-OCS, RWEC-RI
Invertebrates
Longfin inshore squid Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Northern shortfin squid Adult RWF
Highly Migratory Species
Albacore tuna Juvenile RWF, RWEC-OCS, RWEC-RI
Adult
RWF, RWEC-OCS
Bluefin tuna Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Skipjack tuna Juvenile RWF
Adult RWF, RWEC-OCS, RWEC-RI
Yellowfin tuna Juvenile RWF, RWEC-OCS, RWEC-RI
Adult RWF, RWEC-OCS
Sharks
1

Essential Fish Habitat Assessment Technical Report
38
Table 2.3-4
Species Life Stage Location
Basking shark Neonate, Juvenile, Adult RWF, RWEC-OCS
Blue shark Neonate, Juvenile, Adult RWF, RWEC-OCS
Common thresher shark Neonate, Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Dusky shark Neonate, Juvenile, Adult RWF, RWEC-OCS
Sand tiger shark Neonate, Juvenile RWF, RWEC-OCS, RWEC-RI
Sandbar shark Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Shortfin mako shark Neonate, Juvenile, Adult RWF, RWEC-OCS
Smoothhound shark complex
(Atlantic stock)
Neonate, Juvenile, Adult RWF, RWEC-OCS, RWEC-RI
Spiny dogfish Sub-adult male, Adult female RWF, RWEC-OCS
Sub-adult female, Adult male RWF, RWEC-OCS, RWEC-RI
White shark Neonate RWF, RWEC-OCS, RWEC-RI
Juvenile, Adult RWF, RWEC-OCS
1
The neonate/young-of-the year life stage for shark species is more similar to a juvenile life stage than a larval life stage. Thus, neonate/young-
of-the year is considered to be a “late” life stage for the purpose of this analysis.

Essential Fish Habitat Assessment Technical Report
39
3.0 ENVIRONMENTAL CONSEQUENCES AND PROTECTION
MEASURES
3.1 Impact Assessment
Potential impacts are characterized as direct or indirect and categorized by Project phase. Anticipated impacts are
characterized as short-term or long-term. Consistent with NEPA (40 C.F.R. § 1508.8.), evaluations in this report
consider both detrimental (or negative) and beneficial impacts of the Project.
• Direct or Indirect: Direct effects are those occurring at the same place and time as the initial cause or action.
Indirect effects are those that occur later in time or are spatially removed from the activity.
• Short-term or Long-term Impacts: Short- or long-term impacts do not refer to any defined period. In general,
short-term impacts are those that occur only for a limited period or only during the time required for
construction activities. Impacts that are short-lived, such as noise from routine maintenance work during
operations, may also be short-term if the activity is short in duration and the impact is restricted to a short,
defined period. Long-term impacts are those that are likely to occur on a recurring or permanent basis or
impacts from which a resource does not recover quickly. In general, direct impacts associated with
construction and decommissioning are considered short-term because they will occur within the
approximate 1-year construction phase. Indirect impacts are determined to be either short-term or long-
term depending on if resource recovery may take several years. Impacts associated with Operations &
Maintenance (O&M) are considered long-term because they occur over the life of the Project (i.e., 25 years
per the Lease but could be extended up to 35 years.
• Proposed Environmental Protection Measures – If measures are proposed to avoid or minimize potential
impacts, the impact evaluation included consideration of these environmental protection measures.
Different impact-producing factors (IPFs) may result in varying levels of impact on EFH and the species/life stages
that associate with those habitats. IPFs that could impact EFH include seafloor disturbance, sediment suspension
and deposition, habitat alteration, noise, traffic, lighting, discharges and releases, and trash and debris.
Impacts on EFH vary by habitat, species, and life stage as discussed below, with some species/life stages being
more vulnerable than others. The analysis of impacts on EFH are discussed separately for the RWF and RWEC in
the following sections. The IPFs are further subdivided into IPFs during the construction and decommissioning
phases of the Project and the O&M phase of the Project. The construction and decommissioning phases are
grouped as activities and equipment usage are similar between these two phases.
3.1.1 Revolution Wind Farm
IPFs resulting in potential impacts on EFH in the RWF area are described in Table 3.1-1 for the construction and
decommissioning phases and in Table 3.1-2 for the O&M phase. At the end of the Project’s operational life, the
Project will be decommissioned in accordance with a detailed decommissioning plan to be developed in compliance
with applicable laws, regulations, and BMPs at that time. All of the impacts associated with these activities are
anticipated to be similar to or less than those described for construction, unless otherwise noted.

Essential Fish Habitat Assessment Technical Report
40
Table 3.1-1 IPFs and Impact Characterization for EFH within the RWF during Construction and Decommissioning
Table 3.1-1
IPF Project Activity
Impact Characterization for on EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Seafloor
Disturbance
Seafloor
preparation
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Impacts on EFH associated with seafloor preparation will primarily be
associated with species that have benthic/demersal early life stages (eggs and larvae, Table
2.3-1) and later life stages (neonates, juveniles, and adults, Table 2.3-2) and prefer the types
of habitats that will be disturbed by seafloor preparation. These activities could cause injury
or mortality to benthic/demersal species, affect their habitat, and disrupt their spawning.
Similarly, seafloor-disturbing activities could result in a small loss of spawning habitat for
Atlantic cod, as studies completed in other regions suggest that cod often demonstrate
spawning site fidelity, returning to the same fine-scale bathymetric locations year after year
to spawn (Hernandez et al. 2013; Siceloff and Howell 2013; Zemeckis et al., 2014a).
However, such homing behavior has not yet been documented amongst individual cod in
southern New England, although conventional tagging studies suggest there is little dispersal
during the winter spawning season (Cadrin et al., 2020). An active Atlantic cod winter
spawning ground has been identified in a broad geographical area that includes Cox Ledge
and surrounding locations (Zemeckis et al. 2014b; Dean et al., 2020). In southern New
England, cod spawn primarily from December through May (Dean et al., 2020; Langan et al.,
2020). There is currently a BOEM funded acoustic telemetry study to better understand the
distribution and habitat use of spawning cod on and around Cox Ledge. Additionally, in a
sampling effort on Cox Ledge by Kovach et al. (2010), the majority of Atlantic cod collected
were in spawning condition. Atlantic cod were not among the consistently prevalent (top 25)
species collected during multi-year sampling by otter trawl and beam trawl in areas that
included Cox Ledge (Malek et al., 2014). Given the availability of similar surrounding habitat,
Project activities are not expected to result in measurable impacts on spawning Atlantic cod.
Non-lethal impacts on EFH are expected to be short-term as the direct effects will cease
after seafloor preparation is completed in a given area and only a small portion of the
available EFH in the area will be disturbed. Impacts on species with designated EFH that
have pelagic early and/or later life stages within the RWF (Tables 2.3-3 and 2.3-4) are
expected to be limited, as pelagic habitats will not be directly affected by seafloor
preparation. However, these species may temporarily vacate the area of disturbance.
Decommissioning activities are expected to cause similar impacts as construction, but these
impacts would be shorter in duration.
Impacts on EFH associated with boulder clearance and related seafloor preparation activities
are expected to be direct and short-term. Boulders relocated during seafloor preparation will
be in new locations and may be in new physical configurations in relation to other boulders.
Concerning these spatial and physical attributes, the boulders are not expected to return to
pre-project conditions. However, relatively rapid (< 1 year) recolonization of these boulders is
expected (Guarinello and Carey, 2020) and will return these boulders to their pre-project
habitat function. Additionally, if relocation results in aggregations of boulders, these new
features could serve as high value refuge habitat for juvenile lobster and fish as they may
provide more complexity and opportunity for refuge than surrounding patchy habitat.
Impact pile driving
and/or vibratory pile
driving/foundation
installation
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Direct impacts on EFH associated with seafloor disturbance from impact pile
driving and/or vibratory pile driving and installation of the foundations (WTG and OSS) and
scour protection are expected to result in similar direct impacts on EFH as seafloor
preparation. Impacts on EFH will be primarily associated with species that have

Essential Fish Habitat Assessment Technical Report
41
Table 3.1-1
IPF Project Activity
Impact Characterization for on EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
benthic/demersal life stages. Impact pile driving and/or vibratory pile driving and foundation
installation could crush benthic/demersal species, particularly eggs and larvae, but also less
mobile older life stages that do not vacate the area. Minimal impacts on EFH are expected
for pelagic species because they are not expected to be near the seafloor during work
activities or subject to crushing or injury through placement of the piles and foundations or
removal of the foundations during decommissioning.
RWF IAC and
OSS-Link Cable
installation
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Direct impacts on EFH associated with the IAC and OSS-Link Cable
installation are expected to result in similar impacts as those discussed for seafloor
preparation, as the IAC will be installed in the same area that will have been disturbed during
seafloor preparation. Decommissioning activities are expected to cause similar impacts as
construction, but these impacts would be shorter in duration.
Additionally, fish eggs and larvae (ichthyoplankton), as well as zooplankton, are expected to
be entrained during hydraulic dredging and jet trencher embedment of the IAC. Jet trencher
and hydraulic dredging equipment use seawater to circulate through hydraulic motors and
jets during installation. Although this seawater is released back into the ocean, it is assumed
that all entrained eggs, larvae, and zooplankton will be killed. These losses are expected to
be low and short-term. A previous assessment conducted for the South Fork Wind Farm
found that the total estimated losses of zooplankton and ichthyoplankton from jet trencher
entrainment were less than 0.001% of the total zooplankton and ichthyoplankton abundance
present in the study area, which encompassed a linearly buffered region of 15 km around the
SFEC and 25 km around the SFWF (INSPIRE Environmental, 2018). Only early life stages
may be affected by jet plow entrainment; later life stages will not be affected.
Limited research has been conducted on the potential impacts of hydraulic dredge
entrainment, but because the volumes of water used by dredges are relatively small, the
entrainment rates of ichthyoplankton are generally thought to be only a small proportion of
the total local production (Reine and Clark, 1998; Reine et al., 1998). Egg and larval life
stages are most likely to experience lethal impacts (Wenger et al., 2017), but later life stages
could also be entrained by hydraulic dredging, with benthic species or species occurring in
high densities having the highest risk (Drabble, 2012; Reine et al., 1998). However, the
entrainment rates for mobile species are considered to be low, and mortality rates of
entrained fish may also be low (Wenger et al., 2017; Drabble, 2012; Reine et al., 1998).
Jet plow and hydraulic dredge entrainment losses are not expected to result in large losses
of zooplankton, ichthyoplankton, or later life stages, and population-level impacts on EFH
species are not anticipated.
Vessel anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Direct impacts on EFH associated with vessel anchoring (including spuds)
are similar to those discussed in seafloor preparation.
Habitat
Alteration
Seafloor
preparation
Impact pile driving
and/or vibratory pile
driving/foundation
installation
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect Impacts: Immediately following impact-producing activities, species with designated
EFH are expected to move back into the area; however, in areas of sediment disturbance
and/or areas with increased sedimentation, demersal/benthic habitat recovery and benthic
infaunal and epifaunal species abundances may take up to 1 to 3 years to recover to pre-
impact levels, based on the results of a number of studies on benthic recovery (e.g., AKRF,
Inc. et al., 2012; Germano et al., 1994; Hirsch et al., 1978; Kenny and Rees, 1994). This
recovery time may result in an indirect, long-term impact on designated EFH for species with
benthic/demersal life stages. Recolonization of sediments by epifaunal and infaunal species

Essential Fish Habitat Assessment Technical Report
42
Table 3.1-1
IPF Project Activity
Impact Characterization for on EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
RWF IAC and
OSS-Link Cable
installation Vessel
anchoring
(including spuds)
and the return of mobile fish and invertebrate species will allow this area to continue to serve
as foraging habitat for EFH species. Pelagic species/life stages may be indirectly affected by
the temporary reduction of benthic forage species, but these impacts are expected to be
small given the availability of similar habitats in the area. Other species may be attracted to
the disruption and prey on dislodged benthic species or other species injured or flushed
during seafloor preparation, IAC and OSS-Link Cable installation, and vessel anchoring
activities.
During decommissioning, foundations and other facilities will be removed to a depth of 15 ft
(4.6 m) below the mudline, unless otherwise authorized by BOEM (30 CFR § 585.910(a)).
Decommissioning would result in the reversal of beneficial effects for species and life stages
that inhabited the structures during the life of the Project. Over time, the disturbed area is
expected to revert to pre-construction conditions, which would result in a beneficial impact
for species and life stages that inhabit soft bottom habitats. Overall, habitat alteration from
decommissioning is expected to cause minimal impacts because similar soft and hard
bottom habitats are already present in and around the RWF (Benthic Assessment; INSPIRE
Environmental, 2020), and the conversion of a relatively small area of habitat is unlikely to
result in substantial effects, as any effect observed will be limited to the immediate vicinity of
the individual structures.
Sediment
Suspension
and
Deposition
Seafloor
preparation
Impact pile driving
and/or vibratory pile
driving/foundation
installation
RWF IAC and
OSS-Link Cable
installation Vessel
anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Seafloor-disturbing activities will result in temporary increases in sediment
suspension and deposition. Sediment transport modeling was performed using RPS’
Suspended Sediment Fate (SSFATE) model, which is a three-dimensional model developed
jointly with the USACE and the Environmental Research Development Center. SSFATE is a
well-known model that has been successfully applied in projects around the globe to
simulate the sediment transport from dredging, cable and pipeline burial operations,
sediment dumping, dewatering operations, and other sediment-disturbing activities. SSFATE
computes TSS concentrations released into the water column and predicts the transport,
dispersion, and settling of the suspended sediment. RPS also performed hydrodynamic
modeling using their 3-dimensional HYDROMAP modeling system to simulate water levels,
circulation patterns, and water volume flux through the study area and to provide
hydrodynamic input (spatially and temporally varying currents) for input into the sediment
transport model. The models, inputs, and results are described in detail in the Hydrodynamic
and Sediment Transport Modeling Report (RPS, 2020).
Several model simulations were run to evaluate the concentrations of suspended sediments,
spatial extent and duration of sediment plumes, and the seafloor deposition resulting from
cable burial activities. The grain size distributions used for modeling were based on samples
collected during field studies performed for the project (Fugro, 2019), which indicate the
sediments are predominately coarse grained in the RWF. For the RWF IAC, a representative
segment of 7,392 ft (2,253 m) of installation was simulated and the modeling results indicate
that sediment plumes with TSS concentrations exceeding the ambient conditions by 100
mg/L could extend up to 853 (260 m) feet from the cable centerline. The plume is expected
to be mostly contained within the bottom of the water column. The model estimated that the
elevated TSS concentrations would be of short duration and expected to return to ambient
conditions in less than 4.8 hours following the cessation of cable burial activities. The
modeling results indicate that sedimentation from IAC burial may exceed 0.4 inch (10 mm) of
deposition up to 197 feet (60 m) from the cable and could cover up to 47 acres (190,202 m
2
).

Essential Fish Habitat Assessment Technical Report
43
Table 3.1-1
IPF Project Activity
Impact Characterization for on EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Sediment suspension and deposition associated with decommissioning activities are
expected to be similar to those from cable burial, but slightly lower in magnitude.
Most marine species have some degree of tolerance to higher concentrations of suspended
sediment because storms, currents, and other natural processes regularly result in increases
in turbidity (MMS, 2009). However, these increases in sediment suspension and deposition
may cause temporary impacts on benthic/demersal EFH. Direct impacts could include
mortality, injury, or temporary displacement of the organisms living on, in, or near the
seafloor. Sediment deposition on eggs or larvae may result in smothering, potentially
resulting in mortality (MMS, 2007). Larger benthic organisms such as shellfish as may be
able to extend feeding tubes and respiratory structures above the sediment (United Kingdom
Department for Business Enterprise and Regulatory Reform, 2008). Maurer et al. (1986)
found that several species of marine benthic infauna (including the clam Mercenaria
mercenaria) exhibited little to no mortality when buried under up to 3 inches (8 cm) of various
types of sediment (from predominantly silt-clay to pure sand). Demersal/benthic early life
stages in or near the area of disturbance would likely be most affected, but these impacts
are not expected to result in population-level effects. Pelagic species could also be affected,
but are expected to temporarily vacate the area to avoid the disturbance and pelagic habitat
quality is expected to quickly return to pre-disturbance levels.
Noise Impact pile driving
and/or vibratory pile
driving
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: To evaluate the levels of underwater noise likely to be generated during
construction, modeling was conducted using JASCO’s Marine Operations Noise model
(MONM) and Full Wave Range Dependent Acoustic Model (FWRAM). These models
combine the outputs of the source model with the spatial and temporal environmental
context (e.g., location, oceanographic conditions, and seabed type) to estimate acoustic
sound fields. For impact hammering of monopile foundations, the physical injury peak sound
pressure threshold of 206 dB (re 1 µPa) for finfish, is predicted to be exceeded within a
maximum range of 337 ft (115 m) from the sound source. Accumulated sound exposure
levels of 187 dB (re 1 µPa
2
∙sec) and 183 dB (re 1 µPa
2
∙sec) were predicted to be exceeded
within a maximum distance of 5.9 miles (9,464 m) and 7.9 miles (12,673 m), respectively.
The finfish behavioral disturbance threshold of 150 dB (re 1 µPa RMS) is predicted to be
exceeded within a maximum distance of 6.6 miles (10,664 m) from the sound source. Full
modeling results are available in the Underwater Acoustic Analysis (Denes et al., 2020).
Sound exposure guidelines and regulations designed to protect finfish are described in terms
of sound pressure levels, but the observable effects of high intensity noise sources on finfish
may actually be caused by exposure to particle motion (Popper and Hawkins, 2018).
However, the particle motion levels associated with a high intensity noise source are difficult
to measure and isolate from sound pressure levels. There is currently very limited
understanding of the potential effects of particle motion on finfish and invertebrates.
All fishes (including elasmobranchs) detect and use particle motion, even for those fishes
that are also sensitive to sound pressure (Popper and Hawkins, 2019). Fishes that do not
possess a swim bladder (sharks, mackerel, flatfish), as well as fishes with a swim bladder
distant from the ear (salmon, tuna, most teleosts) are thought to primarily be sensitive to
particle motion (Hawkins et al., 2020). Fishes with the swim bladder close to the ear (Atlantic
cod, eels) or where the swim bladder is connected to the ear (herrings) are able to detect
sound pressure as well as particle motion (Hawkins et al., 2020). In these finfish, the swim
bladder and other gas-filled organs may act as a type of acoustic transformer, converting

Essential Fish Habitat Assessment Technical Report
44
Table 3.1-1
IPF Project Activity
Impact Characterization for on EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
sound pressure into particle motion (Popper and Hawkins, 2018). The movement of these
organs may indirectly stimulate the otolith structures such that fishes experience particle
motion both from the noise source and from this indirect signal (Popper and Hawkins, 2018).
Cephalopods, including cuttlefish, octopus, and squid species, are likely sensitive to particle
motion rather than sound pressure (e.g. Packard et al., 1990; Mooney et al., 2010), with the
lowest particle motion thresholds reported at 1 to 2 Hz (Packard et al., 1990). Particle motion
thresholds were measured for longfin squid between 100 and 300 Hz, with a threshold of
110 dB re 1 µPa reported at 200 Hz (Mooney et al., 2010). No other studies have measured
particle motion. Cephalopods appear to be particularly sensitive to low frequency sound.
Solé et al. (2017) estimated that trauma onset may begin to occur in cephalopods at sound
pressure levels (SPL
rms
) from 139 to 142 dB re 1 μPa at one-third octave bands centered at
315 Hz and 400 Hz. A recent study found impulsive pile driving noise resulted in a change in
squid (Doryteuthis pealeii) behavior, with squid exhibiting body pattern changes, inking,
jetting, and startle responses (Jones et al., 2020).
Sessile invertebrates such as bivalves may respond to sound exposure by closing their
valves (e.g. Kastelein, 2008; Roberts et al., 2015; Solan et al., 2016) much as they do when
water quality is temporarily unsuitable. In one study, the duration of valve closure was shown
to increase with increasing vibrational strength (Roberts et al., 2015). Clams may respond to
anthropogenic noise by reducing activity and moving to a position above the sediment-water
interface.
In response to noise associated with pile driving at the RWF, it is expected that finfish and
mobile macroinvertebrates would temporarily relocate during construction and would not be
in the areas of greatest acoustic stressors. Slow start (ramp up) of pile driving equipment
would allow mobile species to move out of the area and not be subject to mortality or injury
but they may still experience some direct impact, such as behavioral responses. For
exposed species, noise from impact pile driving and/or vibratory pile driving may temporarily
reduce habitat quality. However, population-level impacts of impact pile driving and/or
vibratory pile driving noise are not expected. Pile driving will be suspended during the winter
months, thereby avoiding potential noise impacts that may disrupt the spawning activity of
Atlantic cod. In conclusion, impact pile driving and/or vibratory pile driving is expected to
result in a direct impact on EFH for both pelagic and demersal life stages, but this impact will
be short-term as once pile driving is completed, the habitat suitability is expected to return to
pre-pile driving conditions.
Vessel noise,
construction
equipment noise,
aircraft noise
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Short-term impacts on EFH could occur due to vessel noise, construction
equipment noise (exclusive of impact pile driving and/or vibratory pile driving noise), and/or
aircraft noise during construction and decommissioning. Sounds created by
mechanical/hydro-jet plows, vessels, or aircraft are continuous or non-impulsive sounds,
which have different characteristics underwater and impacts on marine life. Limited research
has been conducted on underwater noise from mechanical/hydro-jet plows. Generally, the
noise from this equipment is expected to be masked by louder sounds from vessels. Also, as
most noise generated by these pieces of equipment will be below the sediment surface and
associated with the high-pressure jets, noise levels are not expected to result in injury or
mortality on EFH species, but may cause finfish to temporarily vacate the area. The duration
of noise at a given location will be short, as vessels will only be present for a short period at
any given location along the cable corridor.

Essential Fish Habitat Assessment Technical Report
45
Table 3.1-1
IPF Project Activity
Impact Characterization for on EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Helicopters will be used for crew transfers between the WTGs and shore. Underwater noise
associated with helicopters is generally brief as compared with the duration of audibility in
the air (Richardson et al., 1995).
Vessel noise may also cause mobile EFH species to temporarily vacate the area. Vessel
sound source levels have been shown to cause several different effects in behavior, TTS,
auditory masking, and blood chemistry. The most common behavioral responses are
avoidance, alteration of swimming speed and direction, and alteration of schooling behavior
(Vabø et al., 2002; Handegard and Tjøstheim, 2005; Sarà et al., 2007; Becker et al., 2013).
These studies also demonstrated that the behavioral changes generally were temporary or
that fish habituated to the noises. EFH species in the vicinity of Project vessels may be
affected by vessel noise but the duration of the disturbance will occur over a very short
period at any given location.
Direct impacts on EFH may result from a temporary degradation of habitat for species that
vacate the area due to elevated noise levels. However, the noise generated by vessel and
aircrafts will be similar to the range of noise from existing vessel and aircraft traffic in the
region, and are not expected to substantially affect the existing underwater noise
environment.
Discharges
and
Releases
Hazardous
materials spills
Wastewater
discharge
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Routine discharges of wastewater (e.g., gray water or black water) or liquids
(e.g., ballast, bilge, deck drainage, stormwater) may occur from vessels, WTGs, or the OSS
during construction and decommissioning; however, those discharges and releases are not
anticipated to result in impacts because all vessel waste will be offloaded, stored, and
disposed of in accordance with all applicable local, state and federal laws and regulations,
such as the Environmental Protection Agency (EPA) and U.S. Coast Guard (USCG)
requirements for discharges and releases to surface waters. In addition, compliance with
applicable Project-specific management practices and requirements will minimize the
potential for adversely impacting water quality and marine life.
The construction/decommissioning of the RWF is not anticipated to lead to any spills of
hazardous materials into the marine environment. Minor releases of hazardous materials
could result in direct and indirect, short-term impacts on EFH. The impacts of spills are
caused by either the physical nature of the material (e.g., physical contamination and
smothering) or by its chemical components (e.g., toxic effects and bioaccumulation). Minor
releases of hazardous materials could also result in indirect impacts on fish and invertebrate
species if the spilled materials affect their eggs and food sources. Impacts would depend on
the depth and volume of the spill, as well as the properties of the material spilled.
All vessels participating in the construction of the RWF will comply with USCG requirements
for management of onboard fluids and fuels, including maintaining and implementing spill
prevention, control, and countermeasure (SPCC) plans. Vessels will be navigated by trained,
licensed vessel operators who will adhere to navigational rules and regulations and vessels
will be equipped with spill handling materials adequate to control or clean up an accidental
spill. Best management practices (BMPs) for fueling and power equipment servicing will be
incorporated into the Project’s Emergency Response Plan and Oil Spill Response Plan
(ERP/OSRP). Accidental releases are minimized by containment and clean-up measures
detailed in the OSRP. Given these measures and the very low likelihood of an inadvertent
release, impacts on EFH are not anticipated.

Essential Fish Habitat Assessment Technical Report
46
Table 3.1-1
IPF Project Activity
Impact Characterization for on EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Marine Trash and Debris
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: The release of trash and debris into offshore waters potentially may occur
from any on-water activities. Certain types of trash and debris could be accidentally lost
overboard during construction and decommissioning, with subsequent effects on EFH.
USCG and EPA regulations require operators to develop waste management plans, post
informational placards, manifest trash sent to shore, and use special precautions such as
covering outside trash bins to prevent accidental loss of solid materials. Also, BOEM lease
stipulations require adherence to Notice to Lessee (NTL) 2015-G03, which instructs
operators to exercise caution in the handling and disposal of small items and packaging
materials, requires the posting of placards at prominent locations on offshore vessels and
structures, and mandates a yearly marine trash and debris awareness training and
certification process. As such, measures will be implemented prior to and during construction
to avoid, minimize, and mitigate impacts related to trash and debris disposal. Given these
measures, impacts from trash and debris on EFH are not anticipated.
Traffic
See Seafloor Disturbance, Noise, Sediment Suspension and Deposition, and Lighting IPFs.
Lighting Construction and
vessel lighting
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Artificial lighting during construction/decommissioning at the RWF will be
associated with navigational and deck lighting on vessels from dusk to dawn. The response
of fish species to artificial lights is highly variable and depends on a number of factors such
as the species, life stage, and the intensity of the light. Small organisms are often attracted
to lights, which in turn attract larger predators to feed on the prey aggregations. Other
species may avoid artificially illuminated areas. Artificial lighting may disrupt the diel vertical
migration patterns of fish and this may affect species richness and community composition
(Nightingale et al., 2006; Phipps, 2001). It could also increase the risk of predation and
disruption of predator/prey interactions and result in the loss of opportunity for dark-adapted
behaviors including foraging and migration (Orr et al., 2013). Artificial lighting associated with
construction and decommissioning would be temporary and limited relative to the
surrounding areas. Lighting will be limited to the minimum necessary to ensure safety and to
comply with applicable regulations. Additionally, no underwater lighting is proposed. Artificial
lighting is not expected to result in measurable impacts on EFH.
1
Early life stages include eggs and larvae. Late life stages include neonates, juveniles, and adults.

Essential Fish Habitat Assessment Technical Report
47
Table 3.1-2 IPFs and Impact Characterization for EFH within the RWF during Operations and Maintenance
Table 3.1-2
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Seafloor
Disturbance
Foundations
(WTG and OSS)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Seafloor disturbance during O&M of the RWF may occur during non-routine
maintenance of bottom-founded infrastructure (e.g., foundations, scour protection). These
maintenance activities are expected to result in similar impacts on EFH as those discussed
for construction/decommissioning (Table 3.1-1), although the extent of disturbance would be
limited to specific areas.
RWF IAC and
OSS-Link Cable
non-routine O&M
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Minimal impacts on EFH are expected from operation of the IAC and OSS-
Link Cable themselves, as they will be buried beneath the seabed. However, non-routine
maintenance may involve sediment-disturbing activities. These maintenance activities are
expected to result in similar direct impacts on EFH as those discussed for
construction/decommissioning (Table 3.1-1), although the extent of the disturbance would be
limited to specific areas along the cable corridor.
Vessel anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: During O&M, anchoring will be limited to vessels required to be onsite for an
extended duration. Impacts on EFH resulting from potential vessel anchoring during O&M
activities are expected to be similar to those discussed in Table 3.1-1.
Habitat
Alteration
Foundations
RWF IAC and
OSS-Link Cable
non-routine O&M
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect Impacts: Once constructed, the RWF will result in changes to seafloor topography
and hydrodynamics because of the presence of foundations, scour protection, and cable
protection. In previous assessments, offshore structures have not been shown to change the
strength or direction of regional oceanic currents that transport eggs and larvae of marine
fishes (RI CRMC, 2010; DONG Energy et al., 2006). Larval recruitment of EFH species from
the water column is not anticipated to be affected by the RWF structures because the
vertical foundations represent a miniscule surface area within the surrounding waters, and
recruitment is generally influenced by numerous environmental signals other than the
presence of physical structure (including stage of larval development, temperature, prey
availability, and chemical odor of conspecifics) (McManus et al., 2018; Pineda et al., 2007).
Foundations have been hypothesized as serving as attachment sites for eggs of squid and
herrings in the North Sea, but data so far are lacking (Vandendriessche et al., 2016).
Planktonic life stages of EFH species would not be directly affected by the introduction of
foundations and scour protection. The seafloor overlaying the majority of buried IAC and
OSS-Link Cable (where cable protection will not exist) is expected to return to pre-
construction conditions over time and no long-term changes to sediment mobility and
depositional patterns are expected.
The presence of the foundations, associated scour protection, and cable protection may
result in both negative and beneficial indirect impacts on EFH due to conversion of habitat
from primarily soft-bottom to hard-bottom. Habitat conversion is expected to cause a shift in
species assemblages towards those found in rocky reef/rock outcrop habitat; this is known
as the “reef effect” (Wilhelmsson et al., 2006; Reubens et al., 2013). This effect is also well
known from other anthropogenic structures in the sea, such as oil platforms, artificial reefs
piers, and shipwrecks (Claudet and Pelletier, 2004; Wilhelmsson et al., 2006; Seaman, 2007;
Langhamer and Wilhelmsson, 2009).
The use of gravel, boulders, and/or concrete mats will create new hard substrate, and this
substrate is expected to be initially colonized by barnacles, tube-forming species, hydroids,
and other fouling species found on existing hard bottom habitat in the region. Mobile

Essential Fish Habitat Assessment Technical Report
48
Table 3.1-2
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
organisms, such as lobsters and crabs, may also be attracted to and occur in and around the
foundation in higher numbers than surrounding areas. Monopiles attract a range of attached
epifauna and epiflora, including barnacles and filamentous algae (Petersen and Malm,
2006). Jacket foundations (which may be used for the OSS) provide a more complex
structure than monopile foundations, and may increase habitat complexity through more
suitable fouling surfaces and increased protection from predators (MMS, 2009). As these
foundations extend from below the seafloor to above the surface of the water, there is
expected to be a zonation of macroalgae from deeper growing red foliose algae and
calcareous algae, to kelps and other species, including those that may grow in subtidal,
intertidal, and splash zone areas. Foundations and cable protection typically also have
crevices that increase structural complexity of the area and attract finfish and invertebrate
species seeking shelter.
EFH for species that have life stages associated with soft-bottom habitats may experience
long-term impacts, as available habitat will be slightly reduced. EFH for species and life
stages that inhabit hard bottom habitats may experience a beneficial effect, depending on
the quality of the habitat created by the foundations and scour protection, and the quality of
the benthic community that colonizes that habitat. Overall, habitat alteration is expected to
cause minimal impacts because similar soft and hard bottom habitats are already present in
and around the RWF (Benthic Assessment; INSPIRE Environmental, 2020), and the
conversion of a relatively small area of habitat is unlikely to result in substantial effects, as
any “reef effect” observed will be limited to the immediate vicinity of the individual structures.
Given the availability of similar surrounding habitat and the limited area of habitat
conversion, O&M of the RWF is not expected to result in measurable impacts on spawning
Atlantic cod. The potential effects of removal of Project structures during decommissioning
are discussed in Table 3.1-1.
Sediment
Suspension
and
Deposition
RWF IAC and
OSS-Link Cable
Vessel anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Increases in sediment suspension and deposition during the O&M phase will
result from vessel anchoring and non-routine maintenance activities that require exposing
the IAC and/or OSS-Link Cable. Direct impacts on EFH resulting from sediment suspension
and deposition during the O&M phase are expected to be similar to those discussed for the
construction and decommissioning phase (Table 3.1-1), but on a more limited spatial scale.
Noise Vessel and
aircraft noise
Direct, long-
term
Direct,
long-term
Direct, long-
term
Direct, long-
term
Direct Impacts: Impacts on EFH from ship and aircraft (i.e., helicopter) noise during O&M of
the RWF are expected to be similar to those discussed for the construction/decommissioning
phase (Table 3.1-1), though lesser in extent. The noise generated by vessel and aircrafts will
be similar to the range of noise from existing vessel and aircraft traffic in the region, and are
not expected to substantially affect the existing underwater noise environment.
WTG operational
noise
Direct, long-
term
Direct,
long-term
Direct, long-
term
Direct, long-
term
Direct Impacts: The underwater noise levels produced by WTGs are expected to be within
the hearing ranges of fish. Depending on the noise intensity, these noises could disturb or
displace fisheries species within the surrounding area or cause auditory masking (MMS,
2007). Noise levels from operation of the RWF WTGs are not expected to result in injury or
mortality, and finfish may become habituated to the operational noise (Thomsen et al., 2006;
Bergström et al., 2014). Lindeboom et al. (2011) found no difference in the residency times
of juvenile cod around monopiles between periods of WTG operation or when WTGs were
out-of-order. This study also found that sand eels did not avoid the wind farm. In a similar
study, the abundance of cod, eel, shorthorn sculpin, and goldsinny wrasse, were found to be
higher near WTGs, suggesting that potential noise impacts from operation did not override

Essential Fish Habitat Assessment Technical Report
49
Table 3.1-2
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
the attraction of these species to the artificial reef habitat (Bergström et al., 2013). Based on
the available literature, operational noise from the WTGs is expected to have minimal
impacts on EFH.
Electric and
Magnetic
Fields
RWF IAC and
OSS-Link Cable
Direct, long-
term
Direct,
long-term
Direct, long-
term
Direct, long-
term
Direct Impacts: Operation of the WTGs does not generate electric and magnetic fields
(EMF); however, once the IAC and OSS-Link Cables become energized, the cables will
produce a magnetic field, both perpendicularly and in a lateral direction around the cables.
The cable will be shielded and, where feasible, buried beneath the seafloor and will
otherwise be protected. Shielded electrical transmission cables do not directly emit electrical
fields into surrounding areas, but are surrounded by magnetic fields that can cause induced
electrical fields in moving water (Gill et al., 2012). Exposure to EMF could be short- or long-
term, depending on the mobility of the species/life stage.
A modeling analysis of the magnetic fields and induced electric fields anticipated to be
produced during operation of the RWF IAC, OSS-Link Cable, and RWEC was performed and
results are included in the Offshore Electric- and Magnetic-Field Assessment (Exponent,
2020). That assessment also summarizes data from field studies conducted to assess
impacts of EMF on marine organisms. These studies constitute the best source of evidence
to assess the potential impacts on finfish and invertebrate behavior or distribution in the
presence of energized cables.
Compared to fish and elasmobranchs, relatively little is known about the response of marine
invertebrates to EMF. Field surveys on the behavior of large crab species and lobster at
submarine cable sites (Love et al., 2017; Hutchison et al., 2018) indicate that the Project’s
calculated magnetic-field levels are not likely to impact the distribution and movement of
large epibenthic crustaceans. Ancillary data and observations from these field studies also
suggest that cephalopod behavior is similarly unaffected by the presence of 60-Hz AC
cables. Based on the modeling results and existing evidence, the EMF associated with the
cables will be below the detection capability of invertebrate species.
The available laboratory-generated research regarding the effects of 50- or 60-Hz on fish
behavior do not indicate that produced fields will have adverse effects on magnetosensitive
and electrosensitive species. Controlled laboratory studies conducted with eel and salmon
(Richardson et al., 1976; Armstrong et al., 2015; Orpwood et al., 2015) support the
conclusion that EMF produced by 50-75 Hz AC cables do not alter the behavior of
magnetosensitive fish species, indicating that high frequency EMF is not easily detected by
magnetosensitive migratory fish species. Laboratory studies assessing the EMF detection
abilities indicate that the EMF detection ability of elasmobranchs decreases as the source
frequency increases over 20 Hz, and suggest that elasmobranchs are unlikely to easily
detect electric fields produced by 50/60 Hz power sources (Andrianov et al., 1984; Kempster
et al., 2013). In a laboratory study, demersal catshark were exposed to magnetic fields
produced by a 50-Hz source and did not exhibit any significant behavioral changes (Orr,
2016). Field studies have also concluded that energized power cables neither attract nor
repel elasmobranchs (Love et al., 2016). Based on the available information, EMF produced
by 50/60 Hz power sources is unlikely to be detected by elasmobranchs, and is unlikely to
cause changes in elasmobranch behavior or distribution.
Love et al. (2016) conducted a series of surveys between 2010 and 2014 to track fish
populations at both energized and unenergized 60-Hz submarine cables off the California

Essential Fish Habitat Assessment Technical Report
50
Table 3.1-2
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
coast. These studies were designed to assess whether EMF produced by the energized
cable had any in situ effects on the distribution of marine species. Over three years of
observations, no differences in fish communities at energized and unenergized cable sites
were noted, indicating that EMF had no effect on fish distributions, although the physical
structure of the unburied cables did attract a higher number of fish versus sediment bottoms,
creating a “reef effect” (Love et al., 2016). Additionally, multiple fish surveys have been
conducted at existing offshore windfarm sites. Results from these studies strongly indicate
that operating windfarms and cables do not adversely affect the distributions of resident fish
populations. Nearly 10 years of pre- and post-operational data from the Horns Rev Offshore
Wind Farm site near Denmark indicate “no general significant changes in the abundance or
distribution patterns of pelagic and demersal fish” (Leonhard et al., 2011), including species
similar to those expected to inhabit the RWF. Researchers did note an increase in fish
species associated with hard ground and vertical features, especially around WTG footings
(Leonhard et al., 2011).
Based on the modeling results and existing evidence, EMF associated with the IAC and
OSS-Link Cable is not expected to adversely affect the populations or distributions of EFH
species in the Project Area. These conclusions are consistent with the findings of a previous
comprehensive review of the ecological impacts of marine renewable energy projects, where
it was determined that there has been no evidence demonstrating that EMF at the levels
expected from marine renewable energy projects will cause an effect (negative or positive)
on any species (Copping et al., 2016). Moreover, a 2019 BOEM report that assessed the
potential for AC EMF from offshore wind facilities to affect marine populations concluded
that, for the southern New England area, no negative effects are expected for populations of
key commercial and recreational fish species (Snyder et al., 2019). Based on this
information, it is not expected that EFH species will be measurably affected by EMF from the
cables.
Discharges
and
Releases
Hazardous
materials spills
Wastewater
discharge
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: As discussed for the construction/decommissioning phase, routine
discharges of wastewater or liquids (e.g., ballast, bilge, deck drainage, stormwater) are not
anticipated to result in impacts because all vessel waste will be offloaded, stored, and
disposed of in accordance with all applicable local, state and federal regulations. In addition,
compliance with applicable Project-specific management practices and requirements will
minimize the potential for adversely impacting water quality and marine life.
The operation of the RWF is not anticipated to lead to any spills of hazardous materials into
the marine environment. Per the information requirements outlined in 30 CFR 585.626, a list
of solid and liquid wastes generated, including disposal methods and locations, as well as
federally regulated chemical products, is found in the Project’s ERP/OSRP. The WTG and
the OSS will be designed for secondary levels of containment to prevent accidental
discharges of hazardous materials to the marine environment. Most maintenance will occur
inside the WTGs, thereby reducing the risk of a spill, and no oils or other wastes are
expected to be discharged during maintenance activities.
All vessels participating in O&M of the RWF will comply with USCG requirements for
management of onboard fluids and fuels, including maintaining and implementing SPCC
plans. Vessels will be navigated by trained, licensed vessel operators who will adhere to
navigational rules and regulations and vessels will be equipped with spill handling materials
adequate to control or clean up an accidental spill. Best management practices (BMPs) for

Essential Fish Habitat Assessment Technical Report
51
Table 3.1-2
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
fueling and power equipment servicing will be incorporated into the Project’s ERP/OSRP.
Accidental releases will be minimized by containment and clean-up measures detailed in the
OSRP. Given these measures and the very low likelihood of an inadvertent release, potential
impacts of a hazardous material spill on EFH are not anticipated.
Marine Trash and Debris
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: As discussed in Table 3.1-1, vessels will adhere to the USCG and EPA
marine trash regulations, as well as BOEM guidance, and trash and debris generated during
O&M of the RWF will be contained on vessels or at staging areas until disposal at an
approved facility. Measures will be implemented prior to and during construction to avoid,
minimize, and mitigate impacts related to trash and debris disposal. Given these measures,
potential impacts from trash and debris on EFH are not anticipated.
Traffic
See Seafloor Disturbance, Noise, Sediment Suspension and Deposition, and Lighting IPFs.
Lighting RWF operational
lighting
Direct, long-
term
Direct,
long-term
Direct, long-
term
Direct, long-
term
Direct Impacts: Artificial lighting during O&M will be associated with vessels, the WTGs, and
the OSS for operational safety and security purposes. The response of fish species to
artificial lights is highly variable and depends on a number of factors such as the species, life
stage, and the intensity of the light. Small organisms are often attracted to lights, which in
turn attract larger predators to feed on the prey aggregations. Other species may avoid
artificially illuminated areas. However, lighting will be limited to the minimum necessary to
ensure safety and to comply with applicable regulations. Because of the limited area that will
have artificial lighting relative to the surrounding areas, and because no underwater lighting
is proposed, overall impacts on EFH are expected to be minimal.
1
Early life stages include eggs and larvae. Late life stages include neonates, juveniles, and adults.

Essential Fish Habitat Assessment Technical Report
52
3.1.2 Revolution Wind Export Cable
IPFs resulting in potential impacts on EFH associated with the RWEC are described in Table 3.1-3 for the
construction and decommissioning phases and in Table 3.1-4 for the O&M phase. At the end of the Project’s
operational life, the Project will be decommissioned in accordance with a detailed decommissioning plan to be
developed in compliance with applicable laws, regulations, and BMPs at that time. All of the impacts associated
with these activities are anticipated to be similar to or less than those described for construction, unless otherwise
noted. The impacts discussed in this section apply to both the RWEC-OCS and RWEC-RI, though the impacts
would vary slightly by habitat composition, which differs slightly between the nearshore and offshore portions of the
RWEC corridor.

Essential Fish Habitat Assessment Technical Report
53
Table 3.1-3 IPFs and Impact Characterization for EFH for the RWEC during Construction and Decommissioning
Table 3.1-3
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Seafloor
Disturbance
Seafloor
preparation
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Direct impacts on benthic species and life stages from seafloor preparation
are expected to be similar to those discussed in Table 3.1-1, with the exception of shallower
areas being affected as the RWEC-RI nears landfall. These shallower areas are expected to
have slightly different species assemblages than the deeper offshore areas near the RWF.
For example, winter flounder eggs present in the shallow portions of the RWEC-RI corridor
could be affected by seafloor disturbance if construction activities take place during the
spawning period (generally December 15-May 31).
As discussed in Section 2.2, the up-estuary stations sampled during the benthic survey
conducted for the Project were generally characterized by finer substrate, dominated by soft-
sediment fauna, higher turbidity, and more reduced sediments. The mid-bay stations were
characterized by mussel and Crepidula beds with other attached organisms including
barnacles, sponges, and macroalgae. The stations at the mouth of Narragansett Bay and the
stations leading offshore to the 3-mile state water boundary were generally dominated by
soft sediment infauna. The results of the benthic survey (Benthic Assessment; INSPIRE
Environmental, 2020) did not indicate the presence of beds for EFH shellfish species within
the RWEC-RI corridor, however, the mussel and Crepidula beds could serve as foraging or
nursery habitat for certain finfish species. Disturbance of this shellfish bed habitat is not
anticipated to result in population-level effects on EFH species, as only a small area would
be affected, and similar habitat is common within the Bay.
Seafloor preparation is expected to have limited impacts on EFH for species that have
pelagic early or later life stages. Decommissioning activities are expected to cause similar
impacts as construction, but these impacts would be shorter in duration.
RWEC installation
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Direct impacts on EFH associated with the RWEC installation/
decommissioning are expected to result in similar impacts as those for seafloor preparation.
Construction of the RWEC landfall would be accomplished with either HDD methodology. A
cofferdam may be used to allow for a dry environment during construction and for managing
sediment, contaminated soils, and bentonite (from HDD operations). Impacts associated with
the installation of a cofferdam (if necessary) would be similar to those discussed for seafloor
preparation, but on a smaller scale. The cofferdam will be a temporary structure used during
construction only. Therefore, no conversion of habitat is expected, and the cofferdam will be
removed prior to the O&M phase.
In addition, as described in Table 3.1-1, fish eggs and larvae (ichthyoplankton), as well as
zooplankton, are expected to be entrained and killed during hydraulic dredging and jet
trencher embedment of the RWEC. These losses are expected to be very low and short-
term. A previous assessment conducted for the South Fork Wind Farm found that the total
estimated losses of zooplankton and ichthyoplankton from jet trencher entrainment were less
than 0.001% of the total zooplankton and ichthyoplankton abundance present in the study
area, which encompassed a linearly buffered region of 15 km around the SFEC and 25 km
around the SFWF (INSPIRE Environmental, 2018). Limited research has been conducted on
the potential impacts of hydraulic dredge entrainment, but because the volumes of water
used by dredges are relatively small, the entrainment rates of ichthyoplankton are generally
thought to be only a small proportion of the total fish production (Reine and Clark, 1998;

Essential Fish Habitat Assessment Technical Report
54
Table 3.1-3
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Reine et al., 1998). Jet plow and hydraulic dredge entrainment losses are not expected to
result in large losses of zooplankton, ichthyoplankton, or later life stages, and population-
level impacts on EFH species are not anticipated.
A small amount of tidal salt marsh, and coastal beach/dune habitat may be affected during
installation of the RWEC-RI. At this time, multiple landfall options are being considered within
the Landfall Work Area, so it is not possible to quantify the areal extent of temporary or
permanent impacts on these habitats. However, the Landfall Work Area would total up to 2.5
acres and would be sited to avoid and minimize impacts on wetland resources to the
maximum extent practicable. If the Landfall Work Area is situated near the western end of
the Landfall Envelope, use of the HDD method would minimize impacts on coastal habitats.
Disturbance of tidally-influenced habitats could result in a direct, long-term impact on EFH for
species that utilize these habitats, though this impact would be limited given the availability
of similar habitat in the general area. Additionally, the tidal salt marsh at Blue Beach is
located above MHW and is likely infrequently inundated only during extremely high tides and
storm surge events. The perimeter of the salt marsh is mostly composed of invasive
common reed (Phragmites australis) and is unlikely to function as high-quality habitat for
EFH species.
Vessel anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Direct impacts on EFH associated with vessel anchoring (including spuds)
are similar to those discussed in seafloor preparation.
Habitat
Alteration
Seafloor
Preparation
RWEC installation
Vessel anchoring
(including spuds)
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect Impacts: As discussed for the construction/decommissioning of the RWF (Table 3.1-
1), in areas of sediment disturbance and/or areas with increased sedimentation, benthic
habitat recovery and benthic infaunal and epifaunal species abundances may take up to 1 to
3 years to recover to pre-impact levels, based on the results of a number of studies on
benthic recovery (e.g., AKRF, Inc. et al., 2012; Germano et al., 1994; Hirsch et al., 1978;
Kenny and Rees, 1994). This recovery time may result in an indirect, long-term impact on
designated EFH for species with benthic/demersal life stages. Recolonization of sediments
by epifaunal and infaunal species and the return of mobile fish and invertebrate species will
allow this area to continue to serve as foraging habitat for EFH species. Pelagic species/life
stages may be indirectly affected by the temporary reduction of benthic forage species, but
these impacts are expected to be very limited given the availability of similar habitats in the
area. Other species may be attracted to the disruption and prey on dislodged benthic
species or other species injured or flushed during seafloor preparation, RWEC installation,
and vessel anchoring activities.
During decommissioning, facilities will be removed to a depth of 15 ft (4.6 m) below the
mudline, unless otherwise authorized by BOEM (30 CFR § 585.910(a)). Decommissioning
would result in the reversal of beneficial effects for species and life stages that inhabited the
cable protection (concrete mattresses or rock structures) during the life of the Project. Over
time, the disturbed area is expected to revert to pre-construction conditions, which would
result in a beneficial impact for species and life stages that inhabit soft bottom habitats.
Overall, habitat alteration from decommissioning is expected to cause minimal impacts
because similar soft and hard bottom habitats are already present in and around the RWEC
corridor (Benthic Assessment; INSPIRE Environmental, 2020), and the conversion of a
relatively small area of habitat is unlikely to result in substantial effects, as any effect
observed will be limited to the immediate vicinity of the individual structures.

Essential Fish Habitat Assessment Technical Report
55
Table 3.1-3
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Sediment
Suspension
and
Deposition
Seafloor
Preparation
RWEC installation
Vessel anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: As discussed in Table 3.1-1, seafloor-disturbing activities will result in
temporary increases in sediment suspension and deposition. Sediment transport modeling
was performed using RPS’ SSFATE model to evaluate the concentrations of suspended
sediments, spatial extent and duration of sediment plumes, and the seafloor deposition
resulting from Project cable burial activities. The modeling results indicate that sediment
plumes with TSS concentrations exceeding the ambient conditions by 100 mg/L could
extend up to 4,528 feet (1,380 m) from the RWEC-RI centerline in state waters, and up to
1,542 feet (470 m) from RWEC-OCS centerline in federal waters. The plume is expected to
be mostly contained within the bottom of the water column, though in shallower waters it may
occupy most of the water column due to the water depth. For the RWEC-OCS, predicted
TSS concentrations above ambient for any single circuit installation do not persist in any
given location for greater than 24 hours, and in most locations (>75 % of the affected area)
concentrations return to ambient within 8 hours. This maximum was predicted to occur along
a part of the route that will only see one circuit installation. The maximum duration above
ambient along the portion of the RWEC where two circuits will be installed was predicted to
be 14 hours per circuit. This corresponds to a total of 28 hours above ambient, however the
two 14-hour periods will likely be separated by time. For installation of one circuit of the
RWEC-RI, predicted TSS concentrations above ambient do not persist in any given location
for greater than 16.3 hours, and in most locations (>75 % of the affected area)
concentrations return to ambient within 4 hours). For installation of two circuits, the maximum
plume exposure is doubled at 32.6 hours, however, the two 16.3-hour periods will likely be
separated by time. The modeling results indicate that sedimentation from RWEC burial may
exceed 0.4 inch (10 mm) of deposition up to 919 feet (280 m) from the cable centerline in
state waters and up to 328 feet (100 m) in federal waters. This thickness of sedimentation
could cover up to 1,126 acres (4,556,760 m
2
) in state waters, and 1,020 acres (4,127,794
m
2
) in federal waters. For the cable landfall, TSS concentrations exceeding ambient
conditions by 100 mg/L could extend up 580 ft (177 m) from the centerline and plume
concentrations above ambient could persist for 256 hours for the HDD. This duration is
longer relative to the water jet assisted cable installation due to the slower installation rate of
the activity and since both trenching and backfilling for two circuits are included.
Sedimentation greater than 0.4 in (10 mm) may extend up to 509 ft (155 m) from the
centerline and could cover up to 19 acres (76,890 m
2
). The models, inputs, and results are
described in detail in the Hydrodynamic and Sediment Transport Modeling Report
(Exponent, 2020). Sediment suspension and deposition associated with decommissioning
activities are expected to be similar, but slightly lower in magnitude. Similar to those
discussed in Table 3.1-1, direct impacts on EFH from sediment suspension and deposition
are expected to be similar to those discussed for construction of the RWF, with greater
impacts on sessile and slow-moving benthic species/life stages compared to mobile and
pelagic species/life stages.
Winter flounder eggs are a sensitive resource within Narragansett Bay. Previous
experiments have shown that a viable hatching rate of winter flounder eggs is reduced when
the eggs are buried by as little as one half of one egg diameter, approximately 0.05
centimeter of sediment (Berry et al., 2003). In other laboratory experiments, winter flounder
eggs were found to be affected by a sedimentation level of 0.065 centimeter, and almost
complete mortality was observed when deposition exceeded 0.25 centimeter (Berry et al.,

Essential Fish Habitat Assessment Technical Report
56
Table 3.1-3
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
2011), Winter flounder eggs could be affected by construction of the RWEC-RI if
sedimentation is experienced in these shallow waters during the spawning period (generally
December 15 to May 31). Given the high natural mortality that occurs during the early life
history stages, adverse effects of burial at the population level are expected to be limited and
only measurable in the immediate vicinity of the construction workspace. Revolution Wind
will employ best management practices to minimize potential sedimentation impacts on
winter flounder eggs in shallow waters. Revolution Wind will also coordinate with applicable
regulatory agencies to define and comply with seasonal restrictions to minimize impacts on
winter flounder and other sensitive finfish species.
Noise Vibratory pile
driving
(cofferdam)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: The cofferdam at the RWEC landfall, if required, may be installed as either a
sheet piled structure into the sea floor or a gravity cell structure placed on the sea floor using
ballast weight. Sheet pile installation would require the use of a vibratory hammer to drive
the sidewalls and endwalls into the seabed, which may take approximately up to 3 days.
Vibratory devices use oscillatory hammers or spinning counterweights that vibrate the pile
and cause the sediment surrounding the pile to liquefy, allowing the pile to move easily into
or out of the sediment. Vibratory pile driving is considered a continuous low-frequency noise
source because the device continuously vibrates until the pile reached the desired depth.
Vibratory devices generally have sound source levels 10 to 20 dB lower than impact
hammers, and the sound level generated rises relatively slowly (California Department of
Transportation, 2009). Vibratory pile driving associated with the cofferdam is not anticipated
to result in exceedance of the injury threshold for fish, however, noise from pile driving may
temporarily reduce habitat quality, result in behavioral changes, or cause mobile species to
temporarily vacate the area. Noise impacts on EFH species from vibratory pile driving may
result in limited short-term impacts, as the habitat suitability is expected to return to pre-pile
driving conditions shortly after cessation of the pile driving activity.
Vessel noise,
construction
equipment noise,
aircraft noise
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Direct impacts on EFH resulting from vessel, construction equipment, and
aircraft noise during construction and decommissioning are expected to be similar to those
discussed in Table 3.1-1.
Discharges
and
Releases
Hazardous
materials spills
Wastewater
discharges
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Impacts associated with wastewater discharges or an inadvertent release of
hazardous material during construction or decommissioning of the RWEC are expected to be
similar to those discussed in Table 3.1-1.
Marine Trash and Debris
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Impacts associated with marine trash and debris are expected to be similar
to those discussed in Table 3.1-1.
Traffic See Seafloor Disturbance, Noise, Sediment Suspension and Deposition, and Lighting IPFs.
Lighting Vessel and
construction
lighting
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: During construction and decommissioning activities, lighting will be
associated with the vessels that will be installing or decommissioning the RWEC. Direct
impacts on EFH from artificial lighting are expected to be short-term because the vessels are
expected to pass quickly along the RWEC corridor during cable installation. As discussed in

Essential Fish Habitat Assessment Technical Report
57
Table 3.1-3
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Table 3.1-1, artificial lighting associated with cable installation would be temporary and
limited relative to the surrounding areas. Lighting will be limited to the minimum necessary to
ensure safety and to comply with applicable regulations. Additionally, no underwater lighting
is proposed. Impacts on EFH due to artificial lighting are expected to be minimal.
1
Early life stages include eggs and larvae. Late life stages include neonates, juveniles, and adults.

Essential Fish Habitat Assessment Technical Report
58
Table 3.1-4 IPFs and Impact Characterization for EFH for the RWEC during Operations and Maintenance
Table 3.1-4
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
Seafloor
Disturbance
RWEC non-
routine O&M
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Minimal impacts on EFH are expected from operation of the RWEC, as it will
be buried beneath the seabed, where feasible, and will otherwise be protected. Seafloor
disturbance during O&M of the RWEC will be limited to non-routine maintenance that may
require uncovering and reburial of the cables, as well as maintenance of cable protection
where present. These maintenance activities are expected to result in similar direct impacts
on EFH as those discussed for construction/decommissioning (Table 3.1-1), although the
extent of disturbance would be limited to specific areas along the RWEC corridor.
Vessel anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Impacts on EFH resulting from potential vessel anchoring during O&M
activities are expected to be similar to those discussed in Table 3.1-1.
Habitat
Alteration
RWEC O&M
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect,
long-term
Indirect Impacts: Cable protection (e.g., concrete mattresses) may be placed in select areas
along the RWEC. The introduction of engineered concrete mattresses or rock to areas of the
seafloor can cause local disruptions to circulation, currents, and natural sediment transport
patterns, though these impacts as expected to be limited given the miniscule surface area
associated with the cable protection compared to the surrounding waters. Under normal
circumstances, these segments of the RWEC are expected to remain covered as accretion
of sediment covers the cable and associated cable protection (where applicable). In non-
routine situations, these segments may be uncovered, and re-burial might be required (for
buried portions of the RWEC). The seafloor overlaying the majority of buried RWEC (where
cable protection will not exist) is expected to return to pre-construction conditions over time
and no long-term changes to sediment mobility and depositional patterns are expected.
Indirect impacts on EFH associated with O&M activities for the RWEC are expected to result
in similar impacts as those discussed for the IAC and OSS-Link Cable in Table 3.1-1, but will
be limited in spatial extent. The protection of the cable may result in the long-term
conversion of soft-bottom habitat to hard-bottom habitat. Similar to the foundations, this
cable protection may have a long-term impact on EFH for species associated with soft-
bottom habitats and a long-term beneficial impact on EFH for species associated with hard-
bottom habitats, depending on the quality of the habitat created by the cable protection, and
the quality of the benthic community that colonizes that habitat. The potential effects of
removal of Project structures during decommissioning are discussed in Table 3.1-2.
Sediment
Suspension
and
Deposition
RWEC non-
routine O&M
Vessel anchoring
(including spuds)
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Increases in sediment suspension and deposition during the O&M phase
may result from vessel anchoring and non-routine maintenance activities that require
exposing portions of the RWEC. Direct impacts on EFH resulting from sediment suspension
and deposition during the O&M phase are expected to be similar to those discussed for the
construction and decommissioning phase (Table 3.1-1), but on a more limited spatial scale.
Noise Vessel and
aircraft noise
Direct, long-
term
Direct,
long-term
Direct, long-
term
Direct, long-
term
Direct Impacts: Impacts on EFH from ship and aircraft noise during O&M of the RWEC are
expected to be similar to those discussed for the construction/decommissioning phase
(Table 3.1-1), though lesser in extent.
Electric and
Magnetic
Fields
RWEC operations
Direct, long-
term
Direct,
long-term
Direct, long-
term
Direct, long-
term
Direct Impacts: Once the RWEC becomes energized, the cables will produce a magnetic
field, both perpendicularly and in a lateral direction around the cables. The cable will be
shielded, where feasible, and buried beneath the seafloor, and will otherwise be protected.
Shielded electrical transmission cables do not directly emit electrical fields into surrounding
areas, but are surrounded by magnetic fields that can cause induced electrical fields in

Essential Fish Habitat Assessment Technical Report
59
Table 3.1-4
IPF Project Activity
Impact Characterization for EFH
Discussion
Benthic/
Demersal
Early Life
Stages
1
Pelagic
Early Life
Stages
1
Benthic/
Demersal
Late Life
Stages
1
Pelagic
Late Life
Stages
1
moving water (Gill et al., 2012). Exposure to EMF could be short- or long-term, depending on
the mobility of the species. A modeling analysis of the magnetic fields and induced electric
fields anticipated to be produced during operation of the RWF IAC, OSS-Link Cable, and
RWEC was performed and results are included in the Offshore Electric- and Magnetic-Field
Assessment (Exponent, 2020). That assessment also summarizes data from field studies
conducted to assess impacts of EMF on marine organisms. As discussed for the RWF IAC
and OSS-Link Cable in Table 3.1-2, behavioral effects and/or changes in EFH species
abundance and distributions due to EMF are not expected. These conclusions are consistent
with the findings of a previous comprehensive review of the ecological impacts of marine
renewable energy projects, where it was determined that there has been no evidence
demonstrating that EMF at the levels expected from marine renewable energy projects will
cause an effect (negative or positive) on any species (Copping et al., 2016). Moreover, a
2019 BOEM report that assessed the potential for AC EMF from offshore wind facilities to
affect marine populations concluded that, for the southern New England area, no negative
effects are expected for populations of key commercial and recreational fish species (Snyder
et al., 2019). Based on this information, it is not expected that EFH species will be
measurably affected by EMF from the cables.
Discharges
and
Releases
Hazardous
materials spills
Wastewater
discharges
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Impacts associated with wastewater discharges or an inadvertent release of
hazardous material during O&M of the RWEC are expected to be similar to those discussed
in Table 3.1-1.
Marine Trash and Debris
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct,
short-term
Direct Impacts: Impacts associated with marine trash and debris are expected to be similar
to those discussed in Table 3.1-1.
Traffic
See Seafloor Disturbance, Noise, Sediment Suspension and Deposition, and Lighting IPFs.
Lighting Vessel lighting
Direct, long-
term
Direct,
long-term
Direct, long-
term
Direct, long-
term
Direct Impacts: Artificial lighting during O&M of the RWEC will be associated only with
vessels. However, lighting will be limited to the minimum necessary to ensure safety and to
comply with applicable regulations. Because of the limited area that will have artificial lighting
relative to the surrounding areas, and because no underwater lighting is proposed, overall
impacts on EFH are expected to be minimal.
1
Early life stages include eggs and larvae. Late life stages include neonates, juveniles, and adults.

Essential Fish Habitat Assessment Technical Report
60
3.2 Summary of Impacts
3.2.1 Summary of Impacts on EFH from RWF IPFs
Based on the IPFs discussed in Tables 3.1-1 and 3.1-2, species with a completely pelagic lifestyle are generally
expected to be less negatively affected than demersal or benthic species. Overall, during construction, O&M, and
decommissioning of the RWF, impacts on EFH species with benthic/demersal life stages are expected to be
exposed to direct impacts from noise associated with impact pile driving and/or vibratory pile driving of foundations,
other noise sources, seafloor disturbance, and sediment suspension/deposition, and indirect impacts from habitat
alteration. EFH species with pelagic life stages are expected to be exposed to direct impacts from impact pile driving
and/or vibratory pile driving noise and other construction/decommissioning noise sources, and indirect impacts from
habitat alteration. Potential impacts from other IPFs are anticipated to be minimal. Potential long-term impacts may
result from the conversion of soft-bottom habitat to hard-bottom habitat associated with the WTG foundations, scour
protection, and protection of the OSS-Link Cable and IAC. These long-term impacts would be reversed following
decommissioning of the Project. None of the IPFs are expected to result in population-level effects on EFH species,
due to the limited scale and intensity of the Project activities, the availability of similar habitat in the surrounding
area, and the implementation of avoidance, minimization, and mitigation measures.
3.2.1.1 EFH Species Least Likely to Experience Impacts
Of the species with EFH designated within the RWF area, those that are least likely to experience impacts have
both pelagic early and late life stages, only have EFH associated with pelagic environments, and/or do not have
preferred habitat present in the RWF area. They include the species and life stages listed in Table 3.2-1 below.
Table 3.2-1 EFH Species Least Likely to Experience Impacts – RWF
Table 3.2-1
Species Egg Larvae Neonate Juvenile Adult
New England Finfish
Atlantic herring (Clupea harengus)
Pollock (Pollachius virens)
Winter flounder (Pseudopleuronectes americanus)
Witch flounder (Glyptocephalus cynoglossus)
Mid-Atlantic Finfish
Atlantic butterfish (Peprilus triacanthus)
Atlantic mackerel (Scomber scombrus)
Bluefish (Pomatomus saltatrix)
Invertebrates
Northern shortfin squid (Illex illecebrosus)
Highly Migratory Species
Albacore tuna (Thunnus alalunga)
Bluefin tuna (Thunnus thynnus)
Skipjack tuna (Katsuwonus pelamis)
Yellowfin tuna (Thunnus albacares)
Sharks
Basking shark (Cetorhinus maximus)
Blue shark (Prionace glauca)
Common thresher shark (Alopias vulpinus)
Dusky shark (Carcharhinus obscurus)
Sand tiger shark (Carcharias taurus)

Essential Fish Habitat Assessment Technical Report
61
Table 3.2-1
Species Egg Larvae Neonate Juvenile Adult
Sandbar shark (Carcharhinus plumbeus)
Shortfin mako shark (Isurus oxyrinchus)
Smoothhound shark complex (Atlantic stock)
(Mustelus canis)
White shark (Carcharodon carcharias)
3.2.1.2 EFH Species Most Likely to Experience Impacts
Of the species with EFH designated within the RWF area that also have preferred habitat present, those with
benthic/demersal early and/or late life stages are the most likely to experience impacts as a result of construction,
O&M, and/or decommissioning of the RWF. The species and associated life stages most likely to experience some
level of short-term or long-term, direct or indirect impact are listed in Table 3.2-2 below.
Conversion of soft-bottom habitat to hard-bottom habitat associated with the WTGs, scour protection, and protection
of the OSS-Link Cable and IAC may have a long-term beneficial effect species with life stages with a preference
for hard-bottom habitats (e.g., gravel, rock, boulders, artificial reefs), depending on the quality of the newly-created
hard-bottom habitat, and the quality of the benthic community that colonizes that habitat. These species and life
stages that may experience a long-term, beneficial effect are listed in Table 3.2-3.
Note that some species could experience both negative and beneficial impacts at different phases of the Project.
Thus, the same species and life stages may appear in both Table 3.2-2 and Table 3.2-3.
Table 3.2-2 EFH Species Most Likely to Experience Negative Impacts – RWF
Table 3.2-2
Species Egg Larvae Neonate Juvenile Adult
New England Finfish
Atlantic cod (Gadus morhua)
Atlantic wolfish (Anarhichas lupus)
Haddock (Melanogrammus aeglefinus)
Monkfish (Lophius americanus)
Ocean pout (Zoarces americanus)
Red hake (Urophycis chuss)
Silver hake (Merluccius bilinearis)
White hake (Urophycis tenuis)
Windowpane flounder (Scophthalmus aquosus)
Winter flounder (Pseudopleuronectes americanus)
Yellowtail flounder (Limanda ferruginea)
Mid-Atlantic Finfish
Black sea bass (Centropristis striata)
Scup (Stenotomus chrysops)
Summer flounder (Paralichthys dentatus)
Invertebrates
Atlantic sea scallop (Placopecten magellanicus)
Longfin inshore squid (Doryteuthis pealeii)

Essential Fish Habitat Assessment Technical Report
62
Table 3.2-2
Species Egg Larvae Neonate Juvenile Adult
Ocean quahog (Arctica islandica)
Skates
Little skate (Leucoraja erinacea)
Winter skate (Leucoraja ocellata)
Sharks
Spiny dogfish (Squalus acanthias)
1
1
Includes sub-adult males and sub-adult females.
Table 3.2-3 EFH Species That May Experience Beneficial Effects – RWF
Table 3.2-3
Species Egg Larvae Neonate Juvenile Adult
New England Finfish
Atlantic cod (Gadus morhua)
Atlantic wolfish (Anarhichas lupus)
Haddock (Melanogrammus aeglefinus)
Monkfish (Lophius americanus)
Ocean pout (Zoarces americanus)
Pollock (Pollachius virens)
Red hake (Urophycis chuss)
Silver hake (Merluccius bilinearis)
Yellowtail flounder (Limanda ferruginea)
Mid-Atlantic Finfish
Black sea bass (Centropristis striata)
Scup (Stenotomus chrysops)
Invertebrates
Atlantic sea scallop (Placopecten magellanicus)
Longfin inshore squid (Doryteuthis pealeii)
Skates
Little skate (Leucoraja erinacea)
Winter skate (Leucoraja ocellata)
3.2.2 Summary of Impacts on EFH from RWEC IPFs
Based on the IPFs discussed in Tables 3.1-3 and 3.1-4, species with a completely pelagic lifestyle are generally
expected to be less negatively affected than demersal or benthic species. Overall, during construction, O&M, and
decommissioning of the RWEC, impacts on EFH species with benthic/demersal life stages are expected to be
exposed to direct impacts from seafloor disturbance, sediment suspension/deposition, and noise IPFs, and indirect
impacts from habitat alteration. EFH species with pelagic life stages are expected to be exposed to direct impacts
from noise. Potential impacts from other IPFs are anticipated to be minimal. Potential long-term impacts may result
from the conversion of soft-bottom habitat to hard-bottom habitat associated with the protection of the RWEC. These
long-term impacts would be reversed following decommissioning of the Project. None of the IPFs are expected to
result in population-level effects on EFH species, due to the limited scale and intensity of the Project activities, the

Essential Fish Habitat Assessment Technical Report
63
availability of similar habitat in the surrounding area, and the implementation of avoidance, minimization, and
mitigation measures.
3.2.2.1 EFH Species Least Likely to Experience Impacts
Of the species with EFH designated within the RWEC area, those that are least likely to experience impacts have
both pelagic early and late life stages, only have EFH associated with pelagic environments, and/or do not have
preferred habitat present in the RWF area. They include the species and life stages listed in Table 3.2-4 below.
Table 3.2-4 EFH Species Least Likely to Experience Impacts – RWEC
Table 3.2-4
Species Egg Larvae Neonate Juvenile Adult
New England Finfish
Atlantic herring (Clupea harengus)
Pollock (Pollachius virens)
Witch flounder (Glyptocephalus cynoglossus)
Mid-Atlantic Finfish
Atlantic butterfish (Peprilus triacanthus)
Atlantic mackerel (Scomber scombrus)
Bluefish (Pomatomus saltatrix)
Highly Migratory Species
Albacore tuna (Thunnus alalunga)
Bluefin tuna (Thunnus thynnus)
Skipjack tuna (Katsuwonus pelamis)
Yellowfin tuna (Thunnus albacares)
Sharks
Basking shark (Cetorhinus maximus)
Blue shark (Prionace glauca)
Common thresher shark (Alopias vulpinus)
Dusky shark (Carcharhinus obscurus)
Sand tiger shark (Carcharias taurus)
Sandbar shark (Carcharhinus plumbeus)
Shortfin mako shark (Isurus oxyrinchus)
Smoothhound shark complex (Atlantic stock)
(Mustelus canis)
White shark (Carcharodon carcharias)
3.2.2.2 EFH Species Most Likely to Experience Impacts
Of the species with EFH designated within the RWEC area that also have preferred habitat present, those with
benthic/demersal early and/or late life stages are the most likely to experience impacts as a result of construction,
O&M, and/or decommissioning of the RWEC. The species and associated life stages most likely to experience
some level of short-term or long-term, direct or indirect impact are listed in Table 3.2-5 below.
Conversion of soft-bottom habitat to hard-bottom habitat associated with the cable protection may have a long-term
beneficial effect on species with life stages with a preference for hard-bottom habitats (e.g., gravel, rock, boulders,
artificial reefs), depending on the quality of the newly-created hard-bottom habitat, and the quality of the benthic
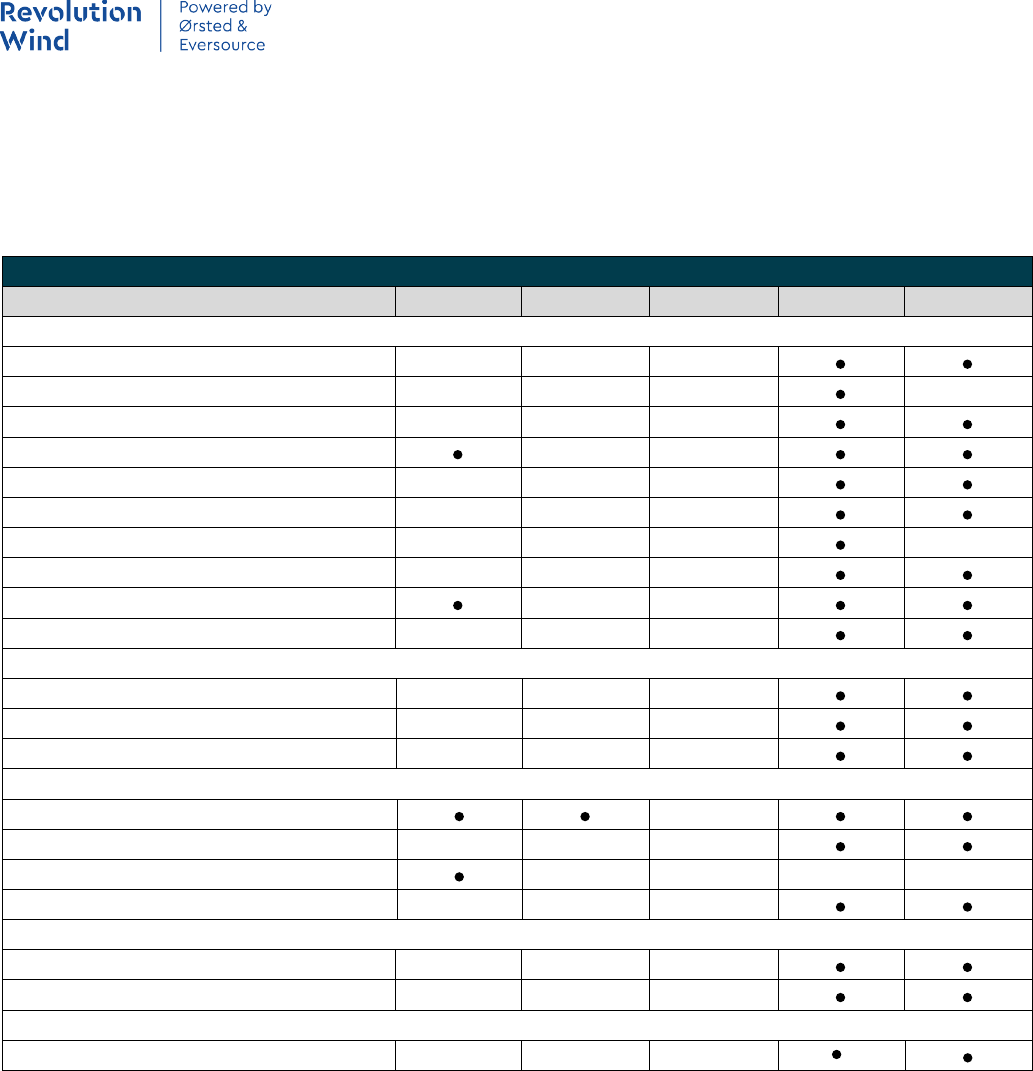
Essential Fish Habitat Assessment Technical Report
64
community that colonizes that habitat. These species and life stages that may experience a long-term, beneficial
effect are listed in Table 3.2-6.
Note that some species could experience both negative and beneficial impacts at different phases of the Project.
Thus, the same species and life stages may appear in both Table 3.2-5 and Table 3.2-6.
Table 3.2-5 EFH Species Most Likely to Experience Negative Impacts – RWEC
Table 3.2-5
Species Egg Larvae Neonate Juvenile Adult
New England Finfish
Atlantic cod (Gadus morhua)
Haddock (Melanogrammus aeglefinus)
Monkfish (Lophius americanus)
Ocean pout (Zoarces americanus)
Red hake (Urophycis chuss)
Silver hake (Merluccius bilinearis)
White hake (Urophycis tenuis)
Windowpane flounder (Scophthalmus aquosus)
Winter flounder (Pseudopleuronectes americanus)
Yellowtail flounder (Limanda ferruginea)
Mid-Atlantic Finfish
Black sea bass (Centropristis striata)
Scup (Stenotomus chrysops)
Summer flounder (Paralichthys dentatus)
Invertebrates
Atlantic sea scallop (Placopecten magellanicus)
Atlantic surfclam (Spisula solidissima)
Longfin inshore squid (Doryteuthis pealeii)
Ocean quahog (Arctica islandica)
Skates
Little skate (Leucoraja erinacea)
Winter skate (Leucoraja ocellata)
Sharks
Spiny dogfish (Squalus acanthias)
1
1
Includes sub-adult males and sub-adult females.

Essential Fish Habitat Assessment Technical Report
65
Table 3.2-6 EFH Species That May Experience Beneficial Effects – RWEC
Table 3.2-6
Species Egg Larvae Neonate Juvenile Adult
New England Finfish
Atlantic cod (Gadus morhua)
Haddock (Melanogrammus aeglefinus)
Monkfish (Lophius americanus)
Ocean pout (Zoarces americanus)
Pollock (Pollachius virens)
Red hake (Urophycis chuss)
Silver hake (Merluccius bilinearis)
Yellowtail flounder (Limanda ferruginea)
Mid-Atlantic Finfish
Black sea bass (Centropristis striata)
Scup (Stenotomus chrysops)
Invertebrates
Atlantic sea scallop (Placopecten magellanicus)
Longfin inshore squid (Doryteuthis pealeii)
Skates
Little skate (Leucoraja erinacea)
Winter skate (Leucoraja ocellata)
3.3 Proposed Environmental Protection Measures
To ensure that impacts associated with the RWF and RWEC are minimized, Revolution Wind will implement the
following environmental protection measures to reduce potential impacts on finfish and EFH. These measures are
based on protocols and procedures successfully implemented for similar offshore projects.
• To the extent feasible, installation of the IACs, OSS-Interlink Cable, and RWEC will occur using equipment
such mechanical cutter, mechanical plow, or jet plow.
• To the extent feasible, the IAC, OSS-Link Cable, and RWEC will target a burial depth of 4 to 6 ft (1.2 to 1.8
m) below seabed. The target burial depth will be determined based on an assessment of seabed conditions,
seabed mobility, the risk of interaction with external hazards such as fishing gear and vessel anchors, and
a site-specific Cable Burial Risk Assessment.
• Dynamic Positioning (DP) vessels will be used for installation of the IAC, OSS-Link Cable, and RWEC to
the extent practicable. DP vessels minimize seafloor impacts, as compared to use of a vessel relying on
multiple anchors.
• A plan for vessels will be developed prior to construction to identify no-anchor areas to avoid documented
sensitive resources.
• Revolution Wind is committed to collaborative science with the commercial and recreational fishing
industries pre-, during, and post-construction. Fisheries monitoring studies are being planned to assess the
impacts associated with the Project on economically and ecologically important fisheries resources. These
studies will be conducted in collaboration with the local fishing industry and will build upon monitoring efforts
being conducted by affiliates of Revolution Wind at other wind farms in the region.

Essential Fish Habitat Assessment Technical Report
66
• Revolution Wind will require all construction and operations vessels to comply with regulatory requirements
related to the prevention and control of spills and discharges.
• Accidental spill or release of oils or other hazardous materials offshore will be managed through the
Project’s ERP/OSRP.
• A ramp-up or soft-start will be used at the beginning of each pile segment during impact pile driving and/or
vibratory pile driving to provide additional protection to mobile species in the vicinity by allowing them to
vacate the area prior to the commencement of pile-driving activities.
• Construction and operational lighting will be limited to the minimum necessary to ensure safety and
compliance with applicable regulations.
• All vessels will comply with USCG and EPA regulations that require operators to develop waste
management plans, post informational placards, manifest trash sent to shore, and use special precautions
such as covering outside trash bins to prevent accidental loss of solid materials. Vessels will also comply
with BOEM lease stipulations that require adherence to Notice to Lessee (NTL) 2015-G03, which instructs
operators to exercise caution in the handling and disposal of small items and packaging materials, requires
the posting of placards at prominent locations on offshore vessels and structures, and mandates a yearly
marine trash and debris awareness training and certification process.
4.0 CONCLUSIONS
Project-related impacts on EFH would vary for different species and life stages based on several factors including
their lifestyle, degree of dependence on the substrate, diet, habitat preferences, and the amount of suitable habitat
present in the area. Most of the potential impacts on EFH will be temporary and reversible as natural processes are
expected to return the disturbed areas to pre-construction conditions apart from new manmade structures on the
seafloor and in the water column. In addition, the extent of anticipated habitat impact is small relative to the
availability of similar habitat in the region.
Construction impacts will largely be associated with the disturbance of benthic habitats in the Project Area. Based
on the results of a number of studies on benthic recovery (e.g., AKRF, Inc. et al., 2012; Germano et al., 1994; Hirsch
et al., 1978; Kenny and Rees, 1994), the affected benthic communities in the disturbed area are expected to re-
establish within 1 to 3 years as native assemblages recolonize the affected area or a new community develops as
a result of immigration of organisms from nearby areas or from larval settlement. Regardless of foundation type(s)
installed, existing habitats will be converted to hard substrate with installation of the WTG foundations (inclusive of
scour protection) and with the installation of cable protection along the IAC, OSS-Link Cable, and RWEC. However,
following construction, these areas of new hard substrate may be suitable for colonization by sessile benthic species
and may provide additional habitat for fish and invertebrate species that inhabit hard bottom habitats. Beneficial
effects for these species would be dependent on their habitat preferences, the quality of the newly-created hard-
bottom habitat, and the quality of the benthic community that colonizes the new habitat. These long-term impacts
would be reversed following decommissioning of the Project. Additional impacts on EFH from operations and
maintenance of the RWF and RWEC would be primarily associated with routine and non-routine maintenance
activities that may require excavation of sediment within a small area. The temporary displacement of these
sediments would impact benthic and demersal EFH in the vicinity, but the impact would be limited considering the
small area affected and the long period of time between maintenance activities. Operational impacts of vessel noise,
traffic, and lighting are considered to be minimal relative to existing marine use activities in the area.
Decommissioning activities associated with the Project, similar to construction activities, will result in temporary
disturbances to EFH and EFH species, but effects and recovery rates are expected to be similar to those described
for construction.

Essential Fish Habitat Assessment Technical Report
67
The overall impacts on EFH associated with the construction, operation, and decommissioning of the RWF and
RWEC are considered to be limited and are not likely to result in population-level effects on EFH species or life
stages.
5.0 REFERENCES
Adams, C.F. 2018. Butterfish 2017 Stock Assessment Update. Northeast Fisheries Science Center Reference
Document 18-05. 36 p. Accessed July 2019. https://repository.library.noaa.gov/view/noaa/17246
.
AKRF, Inc., AECOM, and A. Popper. 2012. Essential Fish Habitat Assessment for the Tappan Zee Hudson River
Crossing Project.
Alade, L. and M. Traver. 2018. 2017 Northern and Southern Silver Hake and Red Hake Stock Assessment
Update Report. Northeast Fisheries Science Center Reference Document 18-02. 71 p. Accessed July 2019.
https://www.nefsc.noaa.gov/publications/crd/crd1802/
.
Andrianov, Y., G.R. Broun, O.B. Il'inskii, and V.M. Muraveiko. 1984. Frequency characteristics of skate
electroreceptive central neurons responding to electrical and magnetic stimulation. Neurophysiology 16.4:
364−369.
Armstrong, J.D., D.C. Hunter, R.J. Fryer, P. Rycroft, and J.E. Orpwood. 2015. Behavioural Responses of Atlantic
Salmon to Mains Frequency Magnetic Fields. Scottish Marine and Freshwater Science 6:9.
Atlantic States Marine Fisheries Commission (ASMFC). 2019a. Black Sea Bass. Accessed July 2019.
http://www.asmfc.org/species/black-sea-bass
.
Atlantic States Marine Fisheries Commission (ASMFC). 2019b. Bluefish. Accessed July 2019.
http://www.asmfc.org/species/bluefish
.
Atlantic States Marine Fisheries Commission (ASMFC). 2019c. Scup. Accessed July 2019.
http://www.asmfc.org/species/scup
.
Atlantic States Marine Fisheries Commission (ASMFC). 2019d. Summer Flounder. Accessed July 2019.
http://www.asmfc.org/species/summer-flounder
.
Atlantic States Marine Fisheries Commission (ASMFC). 2019e. Spiny Dogfish. Accessed July 2019.
http://www.asmfc.org/species/spiny-dogfish
.
Becker, A., A.K. Whitfield, P.D. Cowley, J. Järnegren, and T.F. Næsje. 2013. Does boat traffic cause
displacement of fish in estuaries? Marine Pollution Bulletin 75(1):168–173.
Bergström, L., L. Kautsky, T. Malm, R. Rosenberg, M. Wahlberg, N.Å. Capetillo, and D. Wilhelmsson. 2014.
Effects of offshore wind farms on marine wildlife – a generalized impact assessment. Environmental
Research Letters 9(3):1-12.
Bergström, L., F. Sundqvist, and U. Bergström. 2013. Effects of an offshore wind farm on temporal and spatial
patterns in the demersal fish community. Marine Ecology Progress Series 485: 199−210.
Berry, W., N. Rubinstein, B. Melzian, and B. Hill. 2003. The Biological Effects of Suspended and Bedded
Sediment (SABS) in Aquatic Systems: A Review. Internal U.S. Environmental Protection Agency Report. 20
August 2003.
Berry, W.J., N.I. Rubinstein, E.K. Hinchey, G. Klein-McPhee, and D. Clarke. 2011. Assessment of dredge-induced
sedimentation effects on winter flounder (Pseudopleuronectes americanus) hatching success: results of

Essential Fish Habitat Assessment Technical Report
68
laboratory investigations. Proceedings of the WEDA XXXI Technical Conference and TAMU 42 Dredging
Seminar.
Cadrin, S.X., D.R. Zemeckis, M.J. Dean, and J. Cournane. 2020. Applied Markers. In: An Interdisciplinary Review
of Atlantic Cod (Gadus morhua) Stock Structure in the Western North Atlantic Ocean. R.S. McBride and
R.K. Smedbol, eds. NOAA Tech Memo NMFS-NE-XXX.
California Department of Transportation. 2009. Technical Guidance for Assessment and Mitigation of the
Hydroacoustic Effects of Pile Driving on Fish. 298 pp.
Cargnelli, L.M., S.J. Griesbach, P.L. Berrien, W.W. Morse, and D.L. Johnson. 1999a. Essential fish habitat source
document: Haddock, Melanogrammus aeglefinus, life history and habitat characteristics. NOAA Tech Memo
NMFS-NE-128. 31 p. Accessed July 2019.
https://www.nefsc.noaa.gov/nefsc/publications/tm/tm128/tm128.pdf
.
Cargnelli, L.M., S.J. Griesbach, D.B. Packer, P.L. Berrien, D.L. Johnson, and W.W. Morse. 1999b. Essential Fish
Habitat Source Document: Pollock, Pollachius virens, Life History and Habitat Characteristics. NOAA Tech
Memo NMFS-NE-131. 38 p. Accessed July 2019.
https://www.nefsc.noaa.gov/nefsc/publications/tm/tm131/tm131.pdf
.
Cargnelli, L.M., S.J. Griesbach, D.B. Packer, P.L. Berrien, W.W. Morse, and D.L. Johnson. 1999c. Essential Fish
Habitat Source Document: Witch Flounder, Glyptocephalus cynoglossus, Life History and Habitat
Characteristics. NOAA Tech Memo NMFS-NE-139. 38 p. Accessed July 2019.
https://www.nefsc.noaa.gov/nefsc/publications/tm/tm139/tm139.pdf
.
Cargnelli, L.M., S.J. Griesbach, D.B. Packer, and E. Weissberger. 1999d. NOAA Tech Memo NMFS-NE-142. 22
p. Accessed July 2019. https://www.nefsc.noaa.gov/nefsc/publications/tm/tm142/tm142.pdf
.
Cargnelli, L.M., S.J. Griesbach, D.B. Packer, and E. Weissberger. 1999e. Essential Fish Habitat Source
Document: Ocean Quahog, Arctica islandica, Life History and Habitat Characteristics. NOAA Tech Memo
NMFS-NE-148. 20 p. Accessed July 2019.
https://www.nefsc.noaa.gov/nefsc/publications/tm/tm148/tm148.pdf
.
Carloni, J.T., R. Wahle, P. Geoghegan and E. Bjorkstedt. 2018. Bridging the spawner-recruit disconnect: trends in
American lobster recruitment linked to the pelagic food web. Bulletin of Marine Science 94(3): 719−735.
Chang, S., W.W. Morse, and P.L. Berrien. 1999a. Essential Fish Habitat Source Document: White Hake,
Urophycis tenuis, Life History and Habitat Characteristics. NOAA Tech Memo NMFS-NE-136. 32 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/tm/tm136/tm136.pdf
.
Chang, S., P.L. Berrien, D.L. Johnson, and W.W. Morse. 1999b. Essential Fish Habitat Source Document:
Windowpane, Scophthalmus aquosus, Life History and Habitat Characteristics. NOAA Tech Memo NMFS-
NE-137. 40 p. Accessed July 2019. https://www.nefsc.noaa.gov/nefsc/publications/tm/tm137/tm137.pdf
.
Claudet, J., and D. Pelletier. 2004. Marine protected areas and artificial reefs: a review of the interactions
between management and scientific studies. Aquatic Living Resources 17: 129−138.
Collette, B.B. and G. Klein-MacPhee, ed. 2002. Bigelow and Schroeder’s Fishes of the Gulf of Maine. 3
rd
Edition.
Washington, DC: Smithsonian Institution Press.
Collie, J.S. and J. King. 2016. Spatial and Temporal Distributions of Lobsters and Crabs in the Rhode Island
Massachusetts Wind Energy Area. Sterling, Virginia: 58 p.
Collie, J.S., A.D. Wood, and H.P. Jeffries. 2008. Long-term shifts in the species composition of a coastal fish
community. Canadian Journal of Fisheries and Aquatic Sciences 65(7), 1352−1365.

Essential Fish Habitat Assessment Technical Report
69
Copping A., N. Sather, L. Hanna, J. Whiting, G. Zydlewski, G. Staines, A. Gill, I. Hutchison, A. O’Hagan, T. Simas,
J. Bald, C. Sparling, J. Wood, and E. Masden. 2016. Annex IV 2016 State of the Science Report:
Environmental Effects of Marine Renewable Energy Development Around the World.
Cross, J.N., C.A. Zetlin, P.L. Berrien, D.L. Johnson, and C. McBride. 1999. Essential Fish Habitat Source
Document: Butterfish, Peprilus triacanthus, Life History and Habitat Characteristics. NOAA Tech Memo
NMFS-NE-145. 50 p. Accessed July 2019. https://www.nefsc.noaa.gov/publications/tm/tm145/tm145.pdf
.
Dean, M., G. DeCelles, D. Zemeckis, and T. Ames. 2020. Early Life History. In: An Interdisciplinary Review of
Atlantic Cod (Gadus morhua) Stock Structure in the Western North Atlantic Ocean. R.S. McBride and R.K.
Smedbol, eds. NOAA Tech Memo NMFS-NE-XXX. June 2020.
Denes, S.L., M.J. Weirathmueller, and E.T. Kusel. 2020. Revolution Wind Underwater Acoustic Analysis: Impact
Pile Driving during Turbine Foundation Installation. Document 01935, Revision 7 v3.0. Technical report by
JASCO Applied Sciences for Revolution Wind, LLC, Providence, R.I.
DONG Energy, Vattenfall, The Danish Energy Authority, and The Danish Forest and Nature Agency. 2006.
Danish Offshore Wind: Key Environmental Issues. November 2006.
Drabble, R. 2012. Projected entrainment of fish resulting from aggregate dredging. Marine Pollution Bulletin, 64:
373–381.
Exponent. 2020. Offshore Electric- and Magnetic-Field Assessment. Prepared for Revolution Wind, LLC.,
Providence, R.I. by Exponent, Bowie, MD.
Fergusson, I., L.J.V. Compagno, and M. Marks. 2009. Carcharodon carcharias. The IUCN Red List of Threatened
Species 2009: e.T3855A10133872. Accessed July 2019.
https://www.iucnredlist.org/species/3855/10133872
.
Fisheries and Oceans Canada. 2018a. Atlantic Wolffish. Accessed July 2019. https://www.dfo-mpo.gc.ca/species-
especes/profiles-profils/wolffish-loup-at-eng.html.
Fisheries and Oceans Canada. 2018b. Blue Shark. Accessed July 2019. https://www.dfo-mpo.gc.ca/species-
especes/profiles-profils/blueshark-requinbleu-eng.html.
Fisheries and Oceans Canada. 2018c. Dusky Shark. Accessed July 2019. https://www.dfo-mpo.gc.ca/species-
especes/profiles-profils/duskyshark-requinobscur-eng.html.
Fowler, S.L. 2009. Cetorhinus maximus. The IUCN Red List of Threatened Species 2009: e.T4292A10763893.
Accessed July 2019. https://www.iucnredlist.org/species/4292/10763893
.
Fugro USA Marine, Inc. 2019. Geophysical Survey, Shallow Hazards and Site Characterization Report, North
Reconnaissance Area, OCS-A 0486 Lease, Offshore NY/RI/MA, Atlantic OCS. Prepared for Deepwater
Wind, LLC. Prepared for Deepwater Wind, LLC, Providence, RI. Prepared by Fugro USA Marine, Inc.
Norfolk, VA. 19 April 2019.
Germano, J., J. Parker, and J. Charles. 1994. Monitoring cruise at the Massachusetts Bay Disposal Site, August
1990. DAMOS Contribution No. 92. U.S. Army Corps of Engineers, New England Division. Waltham,
Massachusetts.
Gill, A.B., M. Bartlett, and F. Thomsen. 2012. Potential interactions between diadromous fishes of U.K.
conservation importance and the electromagnetic fields and subsea noise from marine renewable energy
developments. Journal of Fish Biology 81: 664–695.
Groner, M.L., J.D. Shields, D.F. Landers, J. Swenarton, and J.M. Hoenig. 2018. Rising Temperatures, Molting
Phenology, and Epizootic Shell Disease in the American Lobster. American Naturalist 192(5): E163-E177.

Essential Fish Habitat Assessment Technical Report
70
Guarinello, M.L., Carey, D.A. 2020. Multi-modal Approach for Benthic Impact Assessments in Moraine Habitats: a
Case Study at the Block Island Wind Farm. Estuaries and Coasts.
https://doi.org/10.1007/s12237-020-
00818-w
Guida, V., A. Drohan, H. Welch, J. McHenry, D. Johnson, V. Kentner, J. Brink, D. Timmons, E. Estela-Gomez.
2017. Habitat Mapping and Assessment of Northeast Wind Energy Areas. Sterling, VA: US Department of
the Interior, Bureau of Ocean Energy Management. OCS Study BOEM 2017-088. 312 p.
Handegard, N.O., and D. Tjøstheim. 2005. When fish meet a trawling vessel: Examining the behaviour of gadoids
using a free-floating buoy and acoustic split-beam tracking. Canadian Journal of Fisheries and Aquatic
Sciences 62(10): 2409–2422.
Hare, J.A., W.E. Morrison, M.W. Nelson, M.M. Stachura, E.J. Teeters, R.B. Griffis, and C.A. Griswold. 2016. A
Vulnerability Assessment of Fish and Invertebrates to Climate Change on the Northeast US Continental
Shelf. PLoS One 11(2), 30.
Hart, D.R. and A.S. Chute. 2004. Essential Fish Habitat Source Document: Sea Scallop, Placopecten
magellanicus, Life History and Habitat Characteristics. NOAA Tech Memo NMFS-NE-189. 32 p. Accessed
July 2019. https://www.nefsc.noaa.gov/publications/tm/tm189/tm189.pdf
.
Hawkins, A.D., C. Johnson, and A.N. Popper. 2020. How to set sound exposure criteria for fishes. The Journal of
the Acoustical Society of America 147: 1762-1777. Accessed September 2020.
https://asa.scitation.org/doi/10.1121/10.0000907Hendrickson, L.C. 2017. Longfin Inshore Squid
(Doryteuthis (Amerigo) pealeii) Stock Assessment Update for 2017. Accessed July 2019.
https://static1.squarespace.com/static/511cdc7fe4b00307a2628ac6/t/59073cc9be65945087783a84/149364
6537724/Doryteuthis_update_April_2017.pdf.
Hendrickson, L.C. and E.M. Holmes. 2004. Essential Fish Habitat Source Document: Northern Shortfin Squid,
Illex illecebrosus, Life History and Habitat Characteristics. NOAA Tech Memo NMFS-NE-191. 46 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/tm/tm191/tm191.pdf
.
Hernandez, K.M., D. Risch, D.M. Cholewiak, M.J. Dean, L.T. Hatch, W.S. Hoffman, A.N. Rice, D. Zemeckis, and
S.M. Van Parijs. 2013. Acoustic monitoring of Atlantic cod (Gadus morhua) in Massachusetts Bay:
implications for management and conservation. ICES Journal of Marine Science. 70: 628-635.
Hirsch, N.D. L.H. DiSalvo, and R. Peddicord. 1978. Effects of dredging and disposal on aquatic organisms.
Technical Report DS-78-5. U.S. Army Engineer Waterways Experiment Station. Vicksburg, MS. NTIS No.
AD A058 989.
Hutchison, Z.; P. Sigray; H. He, A. Gill, J. King, and C. Gibson. 2018. Electromagnetic Field (EMF) Impacts on
Elasmobranch (shark, rays, and skates) and American Lobster Movement and Migration from Direct
Current Cables. Report by University of Rhode Island, Cranfield University, and FOI (Swedish Defence
Research Agency).
International Commission for the Conservation of Atlantic Tunas (ICCAT). 2014. Report of the 2014 ICCAT East
and West Atlantic Skipjack Stock Assessment Meeting. Accessed July 2019.
https://www.iccat.int/Documents/Meetings/Docs/2014_SKJ_ASSESS_ENG.pdf
.
International Commission for the Conservation of Atlantic Tunas (ICCAT). 2015. Report of the 2015 ICCAT Blue
Shark Stock Assessment Session. Accessed July 2019.
https://www.iccat.int/Documents/SCRS/DetRep/BSH_SA_ENG.PDF
.
International Commission for the Conservation of Atlantic Tunas (ICCAT). 2016a. Report of the 2016 ICCAT
North and South Atlantic Albacore Stock Assessment Meeting. Accessed July 2019.
https://www.iccat.int/Documents/Meetings/Docs/2016_ALB_REPORT_ENG.pdf
.

Essential Fish Habitat Assessment Technical Report
71
International Commission for the Conservation of Atlantic Tunas (ICCAT). 2016b. Report of the 2016 ICCAT
Yellowfin Tuna Stock Assessment Meeting. Accessed July 2019.
https://www.iccat.int/Documents/SCRS/DetRep/YFT_SA_ENG.pdf
.
International Commission for the Conservation of Atlantic Tunas (ICCAT). 2017. Report of the Standing
Committee on Research and Statistics (SCRS). Accessed July 2019.
https://www.iccat.int/Documents/Meetings/Docs/2017_SCRS_REP_ENG.pdf
.
INSPIRE Environmental. 2018. Ichthyoplankton and Zooplankton Assessment – Jet Plow Entrainment Report.
Prepared for CH2M and South Fork Wind Farm.
INSPIRE Environmental. 2020. Benthic Assessment Technical Report. Prepared for Revolution Wind, LLC,
Providence, R.I.
Jaini, M., R.A. Wahle, A.C. Thomas, and R. Weatherbee. 2018. Spatial surface temperature correlates of
American lobster (Homarus americanus) settlement in the Gulf of Maine and southern New England shelf.
Bulletin of Marine Science 94(3): 737–751.
Johnson, D.L., W.W. Morse, P.L. Berrien, and J.J. Vitaliano. 1999. Essential Fish Habitat Source Document:
Yellowtail Flounder, Limanda ferruginea, Life History and Habitat Characteristics. NOAA Tech Memo
NFMS-NE-140. 38 p. Accessed July 2019. https://www.nefsc.noaa.gov/publications/tm/tm140/tm140.pdf
.
Jones, I.T., J.A. Stanley, and T.A. Mooney. 2020. Impulsive pile driving noise elicits alarm responses in squid
(Doryteuthis paeleii). Marine Pollution Bulletin, 150: 110792.
Kastelein, R.A., 2008. Effects of vibrations on the behaviour of cockles (bivalve molluscs). Bioacoustics 17, 74–
75.
Kempster, R.M., N.S. Hart, and S.P. Collin. 2013. Survival of the stillest: predator avoidance in shark embryos.
PLoS One 8(1):e52551.
Kenny, A.J. and H.L. Rees. 1994. The effects of marine gravel extraction on the macrobenthos: Early
postdredging recolonization. Marine Pollution Bulletin 28: 442-447.
Kleisner, K.M., M.J. Fogarty, S. McGee, J.A. Hare, S. Moret, C.T. Perretti, and V.S. Saba. 2017. Marine species
distribution shifts on the US Northeast Continental Shelf under continued ocean warming. Progress in
Oceanography 153: 24–36.
Kovach, A. I., T. S. Breton, D. L. Berlinsky, L. Maceda, and I. Wirgin. 2010. Fine-scale spatial and temporal
genetic structure of Atlantic Cod off the Atlantic coast of the USA. Marine Ecology Progress Series 410:
177–195.Kuffner, A. 2018. Front line of climate change: Black sea bass surge off R.I., new article.
Providence Journal, July 15, 2018. Accessed January, 2020.
https://www.providencejournal.com/news/20180715/front-line-of-climate-change-black-sea-bass-surge-off-
ri.
Langan, J.A., M.C. McManus, D.R. Zemeckis, and J.S. Collie. 2020. Abundance and distribution of Atlantic cod
(Gadus morhua) in a warming southern New England. Fishery Bulletin 118: 145-156.
Langhamer, O., and D. Wilhelmsson. 2009. Colonization of fish and crabs of wave energy foundations and the
effects of manufactured holes – a field experiment. Marine Environmental Research 68(4): 151–157.
Leonhard, S.B., C. Stenberg C, and J.G. Støttrup, eds. 2011. Effect of the Horns Rev 1 Offshore Wind Farm on
Fish Communities: Follow-up Seven Years after Construction. Danish Energy Authority.
Lindeboom, H.J., H.J. Kouwenhoven, M.J.N. Bergman, S. Bouma, S. Brasseur, R. Daan, R.C. Fijn, D. de Haan,
S. Dirksen, R. van Hal, R. Hille Ris Lambers, R. ter Hofstede, K.L. Krijgsveld, M. Leopold, and M. Scheidat.

Essential Fish Habitat Assessment Technical Report
72
2011. Short-term ecological effects of an offshore wind farm in the Dutch coastal zone; a compilation.
Environmental Research Letters 6: 1–13.
Lock, M.C. and D.B. Packer. 2004. Essential Fish Habitat Source Document: Silver Hake, Merluccius bilinearis,
Life History and Habitat Characteristics. NOAA Tech Memo NMFS-NE-186. 78 p. Accessed July 2019.
https://www.nefsc.noaa.gov/publications/tm/tm186/tm186.pdf
.
Love, M.S., M.M. Nishimoto, S. Clark, and A.S. Bull. 2016. Renewable Energy in situ Power Cable Observation.
OCS Study 2016-008. Camarillo, CA: U.S. Department of the Interior, Bureau of Ocean Energy
Management, Pacific OCS Region.
Love, M.S., M.M. Nishimoto, S. Clark, M. McCrea, and A.S. Bull. 2017. Assessing potential impacts of energized
submarine power cables on crab harvests. Continental Shelf Research Dec 1:151: 23–9.
Malek, A., J.S. Collie, and J. Gartland. 2014. Fine scale spatial patterns in the demersal fish and invertebrate
community in a Northwest Atlantic ecosystem. Estuarine, Coastal and Shelf Science 147: 1–10.
Maurer, D., R.T. Keck, J.C. Tinsman, W.A. Leathem, C. Wethe, C. Lord, and T. Church. 1986. Vertical migration
and mortality of marine benthos in dredged material: a synthesis. International Revue des Gesammten
Hydrobiologie 71(1): 49–63.
McBride, R.S., M.K. Tweedie and K. Oliveira. 2018. Reproduction, first-year growth, and expansion of spawning
and nursery grounds of black sea bass (Centropristis striata) into a warming Gulf of Maine. Fishery Bulletin
116(3-4): 323–336.
McManus, M.C., J.A. Hare, D.E. Richardson, and J.S. Collie. 2018. Tracking shifts in Atlantic mackerel (Scomber
scombrus) larval habitat suitability on the Northeast US Continental Shelf. Fisheries Oceanography 27(1):
49–62.
Mid-Atlantic Fishery Management Council and the National Marine Fisheries Service (NOAA Fisheries). 2018.
Squid Amendment: Atlantic Mackerel, Squid, and Butterfish Fishery Management Plan. 224 p. Accessed
July 2019.
https://static1.squarespace.com/static/511cdc7fe4b00307a2628ac6/t/5c113b1f70a6ad290cf75cfd/1544633
161550/20181018_Squid-Amendment-Final+EA.pdf.
Minerals Management Service (MMS). 2007. Programmatic environmental impact statement for alternative
energy development and production and alternate use of facilities on the Outer Continental Shelf – final
environmental impact statement. U.S. Dept. of the Interior, Minerals Management Service, Herndon, VA.
OCS EIS/EA MMS 2007-046.
Minerals Management Service (MMS). 2009. Cape Wind Energy Project Final Environmental Impact Statement
(FEIS). MMS EIS-EA, OCS Publication No. 2008-040. Accessed September 2019.
https://www.boem.gov/Renewable-Energy-Program/Studies/Cape-Wind-FEIS.aspx
.
Mooney, T.A., R.T. Hanlon, J. Christensen-Dalsgaard, P.T. Madsen, D.R. Ketten, and P.E. Nachtigal. 2010.
Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to
low-frequency particle motion and not pressure. Journal of Experimental Biology. 213(21): 3748-3759.
Musick, J.A., R.D., Grubbs, J. Baum, and E. Cortés. 2009a. Carcharhinus obscurus. The IUCN Red List of
Threatened Species 2009: e.T3852A10127245. Accessed July 2019.
https://www.iucnredlist.org/species/3852/10127245
.
Musick, J.A., J.D. Stevens, J.K. Baum, M. Bradai, S. Clò, I. Fergusson, R.D. Grubbs, A. Soldo, M. Vacchi, and
C.M. Vooren. 2009b. Carcharhinus plumbeus. The IUCN Red List of Threatened Species 2009:
e.T3853A10130397. Accessed July 2019. https://www.iucnredlist.org/species/3853/10130397
.

Essential Fish Habitat Assessment Technical Report
73
National Marine Fisheries Service (NOAA Fisheries). 2017. Amendment 10 to the 2006 Consolidated Atlantic
Highly Migratory Species Fishery Management Plan: Essential Fish Habitat. Office of Sustainable Fisheries,
Atlantic Highly Migratory Species Management Division. 442 p. Accessed July 2019.
https://www.habitat.noaa.gov/application/efhinventory/docs/a10_hms_efh.pdf
.
National Marine Fisheries Service (NOAA Fisheries). 2019a. Essential Fish (EFH) Habitat Mapper. Accessed July
2019. https://www.fisheries.noaa.gov/resource/map/essential-fish-habitat-mapper
.
National Marine Fisheries Service (NOAA Fisheries). 2019b. Atlantic Cod. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-cod
.
National Marine Fisheries Service (NOAA Fisheries). 2019c. Atlantic Herring. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-herring
.
National Marine Fisheries Service (NOAA Fisheries). 2019d. Haddock. Accessed July 2019.
https://www.fisheries.noaa.gov/species/haddock
.
National Marine Fisheries Service (NOAA Fisheries). 2019e. Monkfish. Accessed July 2019.
https://www.fisheries.noaa.gov/species/monkfish
.
National Marine Fisheries Service (NOAA Fisheries). 2019f. Atlantic Pollock. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-pollock
.
National Marine Fisheries Service (NOAA Fisheries). 2019g. Red Hake. Accessed July 2019.
https://www.fisheries.noaa.gov/species/red-hake
.
National Marine Fisheries Service (NOAA Fisheries). 2019h. Silver Hake. Accessed July 2019.
https://www.fisheries.noaa.gov/species/silver-hake
.
National Marine Fisheries Service (NOAA Fisheries). 2019i. Winter Flounder. Accessed July 2019.
https://www.fisheries.noaa.gov/species/winter-flounder
.
National Marine Fisheries Service (NOAA Fisheries). 2019j. Yellowtail Flounder. Accessed July 2019.
https://www.fisheries.noaa.gov/species/yellowtail-flounder
.
National Marine Fisheries Service (NOAA Fisheries). 2019k. Butterfish. Accessed July 2019.
https://www.fisheries.noaa.gov/species/butterfish
.
National Marine Fisheries Service (NOAA Fisheries). 2019l. Atlantic Mackerel. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-mackerel
.
National Marine Fisheries Service (NOAA Fisheries). 2019m. Black Sea Bass. Accessed July 2019.
https://www.fisheries.noaa.gov/species/black-sea-bass
.
National Marine Fisheries Service (NOAA Fisheries). 2019n. Bluefish. Accessed July 2019.
https://www.fisheries.noaa.gov/species/bluefish
.
National Marine Fisheries Service (NOAA Fisheries). 2019o. Scup. Accessed July 2019.
https://www.fisheries.noaa.gov/species/scup
.
National Marine Fisheries Service (NOAA Fisheries). 2019p. Summer Flounder. Accessed July 2019.
https://www.fisheries.noaa.gov/species/summer-flounder
.
National Marine Fisheries Service (NOAA Fisheries). 2019q. Atlantic Sea Scallop. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-sea-scallop
.
National Marine Fisheries Service (NOAA Fisheries). 2019r. Atlantic Surfclam. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-surfclam
.

Essential Fish Habitat Assessment Technical Report
74
National Marine Fisheries Service (NOAA Fisheries). 2019s. Longfin Squid. Accessed July 2019.
https://www.fisheries.noaa.gov/species/longfin-squid
.
National Marine Fisheries Service (NOAA Fisheries). 2019t. Ocean Quahog. Accessed July 2019.
https://www.fisheries.noaa.gov/species/ocean-quahog
.
National Marine Fisheries Service (NOAA Fisheries). 2019u. North Atlantic Albacore Tuna. Accessed July 2019.
https://www.fisheries.noaa.gov/species/north-atlantic-albacore-tuna
.
National Marine Fisheries Service (NOAA Fisheries). 2019v. Western Atlantic Bluefin Tuna. Accessed July 2019.
https://www.fisheries.noaa.gov/species/western-atlantic-bluefin-tuna
.
National Marine Fisheries Service (NOAA Fisheries). 2019w. Atlantic Skipjack Tuna. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-skipjack-tuna
.
National Marine Fisheries Service (NOAA Fisheries). 2019x. Atlantic Yellowfin Tuna. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-yellowfin-tuna
.
National Marine Fisheries Service (NOAA Fisheries). 2019y. Winter Skate. Accessed July 2019.
https://www.fisheries.noaa.gov/species/winter-skate
.
National Marine Fisheries Service (NOAA Fisheries). 2019z. Atlantic Common Thresher Shark. Accessed July
2019. https://www.fisheries.noaa.gov/species/atlantic-common-thresher-shark
.
National Marine Fisheries Service (NOAA Fisheries). 2019aa. Atlantic Shortfin Mako Shark. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-shortfin-mako-shark
.
National Marine Fisheries Service (NOAA Fisheries). 2019ab. Atlantic Spiny Dogfish. Accessed July 2019.
https://www.fisheries.noaa.gov/species/atlantic-spiny-dogfish
.
New England Fishery Management Council (NEFMC). 2017. Omnibus Essential Fish Habitat Amendment 2.
Volume 2: EFH and HAPC Designation Alternatives and Environmental Impacts. Accessed July 2019.
https://www.habitat.noaa.gov/protection/efh/efhmapper/oa2_efh_hapc.pdf
.
Nightingale, B., T. Longcore, and C.A. Simenstad. 2006. Artificial night lighting and fishes. In: Ecological
Consequences of Artificial Night Lighting. C. Rich and T. Longcore, eds. Washington, DC: Island Press. pp.
257–276.
Northeast Fisheries Science Center (NEFSC). 2013. 2013 Monkfish Operational Assessment. Northeast Fisheries
Science Center Reference Document 13-23. 116 p. Accessed July 2019.
https://www.nefsc.noaa.gov/publications/crd/crd1323/
.
Northeast Fisheries Science Center (NEFSC). 2015. 60th Northeast Regional Stock Assessment Workshop (60th
SAW) Assessment Report. Northeast Fisheries Science Center Reference Document 15-08. 870 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1508/
.
Northeast Fisheries Science Center (NEFSC). 2016. 61st Northeast Regional Stock Assessment Workshop (61st
SAW) Assessment Summary Report. Northeast Fisheries Science Center Reference Document 16-13. 26
p. Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1613/crd1613.pdf
.
Northeast Fisheries Science Center (NEFSC). 2017a. Operational Assessment of 19 Northeast Groundfish
Stocks, Updated Through 2016. Northeast Fisheries Science Center Reference Document 17-17. 259 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1717/
.
Northeast Fisheries Science Center (NEFSC). 2017b. 62nd Northeast Regional Stock Assessment Workshop
(62nd SAW) Assessment Report. Northeast Fisheries Science Center Reference Document 17-03. 822 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1703/
.

Essential Fish Habitat Assessment Technical Report
75
Northeast Fisheries Science Center (NEFSC). 2017c. Scup Stock Assessment Update for 2017. Accessed July
2019.
https://static1.squarespace.com/static/511cdc7fe4b00307a2628ac6/t/596fb26bc534a5fa937b2c07/1500492
396171/5Scup_2017_Assesssment_Update.pdf.
Northeast Fisheries Science Center (NEFSC). 2017d. 63rd Northeast Regional Stock Assessment Workshop
(63rd SAW) Assessment Report. Northeast Fisheries Science Center Reference Document 17-10. 409 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1710/
.
Northeast Fisheries Science Center (NEFSC). 2018a. 65th Northeast Regional Stock Assessment Workshop
(65th SAW) Assessment Summary Report. Northeast Fisheries Science Center Reference Document 18-
08. 38 p. Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1808/
.
Northeast Fisheries Science Center (NEFSC). 2018b. 64th Northeast Regional Stock Assessment Workshop
(64th SAW) Assessment Summary Report. Northeast Fisheries Science Center Reference Document 18-
03. 27 p. Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1803/
.
Northeast Fisheries Science Center (NEFSC). 2019. 66th Northeast Regional Stock Assessment Workshop (66th
SAW) Assessment Summary Report. Northeast Fisheries Science Center Reference Document 19-01. 40
p. Accessed July 2019. https://www.nefsc.noaa.gov/publications/crd/crd1901/
.
Nye, J.A., J.S. Link, J.A. Hare, and W.J. Overholtz. 2009. Changing spatial distribution of fish stocks in relation to
climate and population size on the Northeast United States continental shelf. Marine Ecology Progress
Series 393: 111–129.
Orpwood, J.E., R.J. Fryer, P. Rycroft, and J.D. Armstrong. 2015. Effects of AC Magnetic Fields (MFs) on
Swimming Activity in European Eels Anguilla. Scottish Marine and Freshwater Science 6:8.
Orr, M. The potential impacts of submarine power cables on benthic elasmobranchs. 2016. Doctoral Dissertation,
The University of Aukland, New Zealand.
Orr, T.L., S. Herz, and D. Oakley. 2013. Evaluation of Lighting Schemes for Offshore Wind Facilities and Impacts
to Local Environments. OCS Study. BOEM 2013-0116.
Packard A., H.E. Karlsen, and O. Sand. 1990. Low frequency hearing in cephalopods. Journal of Comparative
Physiology A. 166: 501–505.
Packer, D.B., S.J. Griesbach, P.L. Berrien, C.A. Zetlin, D.L. Johnson, and W.W. Morse. 1999. Essential Fish
Habitat Source Document: Summer Flounder, Paralichthys dentatus, Life History and Habitat
Characteristics. NOAA Tech Memo NMFS-NE-151. 98 p. Accessed July 2019.
https://www.nefsc.noaa.gov/nefsc/publications/tm/tm151/tm151.pdf
.
Packer, D.B., C.A. Zetlin, and J.J. Vitaliano. 2003a. Essential Fish Habitat Source Document: Little Skate,
Leucoraja erinacea, Life History and Habitat Characteristics. NOAA Tech Memo NMFS-NE-175. 76 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/tm/tm175/tm175.pdf
.
Packer, D.B., C.A. Zetlin, and J.J. Vitaliano. 2003b. Essential Fish Habitat Source Document: Winter Skate,
Leucoraja ocellata, Life History and Habitat Characteristics. NOAA Tech Memo NMFS-NE-179. 68 p.
Accessed July 2019. https://www.nefsc.noaa.gov/publications/tm/tm179/tm179.pdf
.
Pereira, J.J., R. Goldberg. J.J. Ziskowski, P.L. Berrien, W.W. Morse, and D.L. Johnson. 1999. Essential Fish
Habitat Source Document: Winter Flounder, Pseudopleuronectes americanus, Life History and Habitat
Characteristics. NOAA Tech Memo NMFS-NE-138. 48 p. Accessed July 2019.
https://www.nefsc.noaa.gov/publications/tm/tm138/tm138.pdf
.
Petersen J.K. and T. Malm. 2006. Offshore windmill farms: threats to or possibilities for the marine environment.
Ambio 35: 75–80.

Essential Fish Habitat Assessment Technical Report
76
Phipps, G. 2001. Signals maintenance shapes salmon solution. Northwest Region Bulletin. p. 2.
Pineda, J., J.A. Hare, and S. Sponaugle. 2007. Larval Transport and Dispersal in the Coastal Ocean and
Consequences for Population Connectivity. Oceanography 20(3): 22–39.
Pinsky, M.L., B. Worm, M.J. Fogarty, J.L. Sarmiento, and S.A. Levin. 2013. Marine Taxa Track Local Climate
Velocities. Science 341(6151): 1239–1242.
Pollard, D. and A. Smith. 2009. Carcharias taurus. The IUCN Red List of Threatened Species 2009:
e.T3854A10132481. Accessed July 2019. https:// https://www.iucnredlist.org/species/3854/10132481
.
Popper, A.N. and A.D. Hawkins. 2018. The importance of particle motion to fishes and invertebrates. The Journal
of the Acoustical Society of America 143: 470. Accessed September 2020.
https://doi.org/10.1121/1.5021594
Popper, A.N. and A.D. Hawkins. 2019. An overview of fish bioacoustics and the impacts of anthropogenic sounds
on fishes. Journal of Fish Biology 94: 692-713. Accessed September 2020.
https://onlinelibrary.wiley.com/doi/pdf/10.1111/jfb.13948
Reid, R.N., L.M. Cargnelli, S.J. Griesbach, D.B. Packer, D.L. Johnson, C.A. Zetlin, W.W. Morse, and P.L. Berrien.
1999. Essential fish habitat source document: Atlantic herring, Clupea harengus, life history and habitat
characteristics. NOAA Tech Memo NMFS NE 126. Accessed July 2019.
https://www.nefsc.noaa.gov/publications/tm/tm126/tm126.pdf
.
Reine, K.J., D.D. Dickerson, and D.G. Clarke. 1998. Environmental windows associated with dredging operations
(pp. 1– 14). U.S. Army Corps of Engineers, Engineer Research and Development Center, Vicksburg, MS,
Technical Note DOER‐E1.
Reine, K.J. and D.G. Clarke. 1998. Entrainment by hydraulic dredges – A review of potential impacts, Technical
Note DOER‐E1 (pp. 1‐14). U.S. Army Corps of Engineers, Engineer Research and Development Center,
Vicksburg, MS.
Rheuban, J.E., M.T. Kavanaugh and S.C. Doney. 2017. Implications of Future Northwest Atlantic Bottom
Temperatures on the American Lobster (Homarus americanus) Fishery. Journal of Geophysical Research-
Oceans 122(12): 9387–9398.
Rhode Island Coastal Resources Management Council (RI CRMC). 2010. Rhode Island Ocean Special Area
Management Plan. Adopted by the RI CRMC on October 19, 2010. Accessed September 2019.
http://seagrant.gso.uri.edu/oceansamp/documents.html.
Rhode Island Geographic Information System (RIGIS). 2003. Narragansett Bay Estuarine Habitat; nbaywet.
Rhode Island Geographic Information System (RIGIS) Data Distribution System, URL: http://www.rigis.org,
Environmental Data Center, University of Rhode Island, Kingston, Rhode Island. Accessed: 2 October
2014. https://www.rigis.org/datasets/narragansett-bay-estuarine-habitat
Rhode Island Geographic Information System (RIGIS). 2017. Submerged Aquatic Vegetation (2012); SAV16.
Rhode Island Eelgrass Mapping Taskforce. M. Bradley, C. Chaffee, and K. Raposa. Rhode Island
Geographic Information System (RIGIS) Data Distribution System. Environmental Data Center, University of
Rhode Island, Kingston, Rhode Island. Accessed September 2019.
http://www.rigis.org/datasets/submerged-aquatic-vegetation-sav-in-ri-coastal-waters-2016
Richardson, N.E., J.D. McCleave, and E.H. Albert. 1976. Effect of extremely low frequency electric and magnetic
fields on locomotor activity rhythms of Atlantic salmon (Salmo salar) and American eels (Anguilla rostrata).
Environmental Pollution 10(1): 65–76.
Richardson, W.J., C.R. Greene, C.I. Malme, and D.H. Thomson. 1995. Marine Mammals and Noise. San Diego,
California: Academic Press.

Essential Fish Habitat Assessment Technical Report
77
Reubens, J.T., U. Braeckman, J. Vanaverbeke, C. Van Colen, S. Degraer, and M. Vincx. 2013. Aggregation at
windmill artificial reefs: CPUE of Atlantic cod (Gadus morhua) and pouting (Trisopterus luscus) at different
habitats in the Belgian part of the North Sea. Fisheries Research 139: 28–34.
Roberts, L., S. Cheesman, T. Breithaupt, and M. Elliott. 2015. Sensitivity of the mussel Mytilus edulis to substrate-
borne vibration in relation to anthropogenically generated noise. Marine Ecology Progress Series 538: 185-
195.
RPS. 2020. Hydrodynamic and Sediment Transport Modeling Report. Prepared for Revolution Wind, LLC,
Providence, R.I. by RPS, South Kingstown, R.I.
Saba, V.S., S.M. Griffies, W.G. Anderson, M. Winton, M.A. Alexander, T.L. Delworth, and R. Zhang. 2016.
Enhanced warming of the Northwest Atlantic Ocean under climate change. Journal of Geophysical
Research-Oceans 121(1), 118–132.
Sarà, G., J.M. Dean, D. D’Amato, G. Buscaino, A. Oliveri, S. Genovese, S. Ferro, G. Buffa, M. Lo Martire, and S.
Mazzola. 2007. Effect of boat noise on the behaviour of bluefin tuna Thunnus thynnus in the Mediterranean
Sea. Marine Ecology Progress Series 331: 243–253.
Seaman, W. 2007. Artificial habitats and the restoration of degraded marine ecosystems and fisheries.
Hydrobiologia 580: 143−155.
Selden, R.L., R.D. Batt, V.S. Saba, and M.L. Pinsky. 2018. Diversity in thermal affinity among key piscivores
buffers impacts of ocean warming on predator-prey interactions. Global Change Biology 24(1), 117−131.
Siceloff, L. and H. Howell. 2013. Fine-scale temporal and spatial distributions of Atlantic Cod (Gadus morhua) on
a western Gulf of Maine spawning ground. Fisheries Research. Vol. 141. pp. 31–43.
Solan, M., C. Hauton, J.A. Godbold, C.L. Wood, T.G. Leighton, and P. White. 2016. Anthropogenic sources of
underwater sound can modify how sediment-dwelling invertebrates mediate ecosystem properties.
Scientific Reports, 6: 1–9. Nature Publishing Group. http://dx.doi.org/10.1038/srep20540.
Solé, M., P. Sigray, M. Lenoir, M. Van Der Schaar, E. Lalander, and M. André. 2017. Offshore exposure
experiments on cuttlefish indicate received sound pressure and particle motion levels associated with
acoustic trauma. Scientific reports. 7:45899.
South Atlantic Fishery Management Council. 2003. Fishery Management Plan for the Dolphin and Wahoo Fishery
of the Atlantic Including a Final Environmental Impact Statement, Regulatory Impact Review, Initial
Regulatory Flexibility Analysis, and Social Impact Assessment/Fishery Impact Statement.
Southeast Data Assessment and Review (SEDAR). 2016. Update Assessment to SEDAR 21: HMS Dusky Shark.
SEDAR, North Charleston SC. Accessed July 2019.
http://sedarweb.org/docs/suar/Dusky_update_report_2016.pdf
.
Southeast Data Assessment and Review (SEDAR). 2018. SEDAR 56 – South Atlantic Black Seabass
Assessment Report. SEDAR, North Charleston SC. 164 p. Accessed July 2019.
http://sedarweb.org/docs/sar/S56_SA_BSB_SAR_FINAL_4.6.2018.pdf
.
Sosebee, K. 2017. 2016 NE Skate Stock Status Update. Accessed July 2019.
http://s3.amazonaws.com/nefmc.org/4_NEFSC_SkateMemo_July_2017_170922_085135.pdf
.
Steimle, F.W., W.W. Morse, P.L. Berrien, D.L. Johnson, and C.A. Zetlin. 1999a. Essential fish habitat source
document: Ocean pout, Macrozoarces americanus, life history and habitat characteristics. NOAA Tech
Memo NMFS-NE-129; 26 p. Accessed July 2019.
https://www.nefsc.noaa.gov/publications/tm/tm129/tm129.pdf
.

Essential Fish Habitat Assessment Technical Report
78
Steimle, F.W., W.W. Morse, P.L. Berrien, and D.L. Johnson. 1999b. Essential Fish Habitat Source Document:
Red Hake, Urophycis chuss, Life History and Habitat Characteristics. NOAA Tech Memo NFMS-NE-133. 42
p. Accessed July 2019. https://www.nefsc.noaa.gov/nefsc/publications/tm/tm133/tm133.pdf
.
Steimle, F.W., C.A. Zetlin, P.L. Berrien, and S. Chang. 1999c. Essential Fish Habitat Source Document: Black
Sea Bass, Centropristis striata, Life History and Habitat Characteristics. NOAA Tech Memo NMFS-NE-143.
50 p. Accessed July 2019. https://www.nefsc.noaa.gov/nefsc/publications/tm/tm143/tm143.pdf
.
Snyder D.B., W.H. Bailey, K. Palmquist, B.R.T. Cotts, and K.R. Olsen. 2019. Evaluation of Potential EMF Effects
on Fish Species of Commercial or Recreational Fishing Importance in Southern New England. U.S. Dept. of
the Interior, Bureau of Ocean Energy Management, Headquarters, Sterling, VA. OCS Study BOEM 2019-
049.
Thomsen, F., K. Lüdemann, R. Kafemann, and W. Piper. 2006. Effects of offshore wind farm noise on marine
mammals and fish, biota. Hamburg, Germany on behalf of COWRIE Ltd.
United Kingdom Department for Business Enterprise and Regulatory Reform. 2008. Review of Cabling
Techniques and Environmental Effects Applicable to the Offshore Wind Industry. Technical Report 2008.
Vabø, R., K. Olsen, and I. Huse. 2002. The effect of vessel avoidance of wintering Norwegian spring spawning
herring. Fisheries Research 58: 59–77.
Vandendriessche, S., A.M. Ribeiro da Costa, and K. Hostens. 2016. Wind farms and their influence on the
occurrence of ichthyoplankton and squid larvae. Environmental impacts of offshore wind farms in the
Belgian part of the North Sea: Environmental impact monitoring reloaded. Royal Belgian Institute of Natural
Sciences, OD Natural Environment, Marine Ecology and Management Section. S. Degraer, R. Brabant, B.
Rumes and L. E. Vigin, Eds. Pages 117-140.
VHB. 2020. The Delineated Wetlands and Wetland Resource Cover Type. GIS Data provided by VHB on
September 9, 2020.
VHB. 2020. Onshore Natural Resources and Biological Assessment. Prepared for Revolution Wind, LLC,
Providence, R.I. by VHB, Providence, R.I.
Wahle, R.A., L. Dellinger, S. Olszewski and P. Jekielek. 2015. American lobster nurseries of southern New
England receding in the face of climate change. ICES Journal of Marine Science 72: 69–78.
Walsh, H.J., D.E. Richardson, K.E. Marancik, and J.A. Hare 2015. Long-Term Changes in the Distributions of
Larval and Adult Fish in the Northeast U.S. Shelf Ecosystem. PLoS One 10(9): e0137382.
Wenger, A.S., E. Harvey, S. Wilson, C. Rawson, S.J. Newman, D. Clarke, B.J. Saunders, N. Browne, M.J.
Travers, J.L. Mcilwain, P.L.A. Erftemeijer, J.A. Hobbs, D. Mclean, M. Depczynski, and R.D. Evans. 2017. A
critical analysis of the direct effects of dredging on fish. Fish and Fisheries, 18(5): 967–985.
Wilhelmsson D., T. Malm, and M.C. Öhman. 2006. The influence of offshore wind power on demersal fish. ICES
Journal of Marine Science 63: 775–84.
Zemeckis, D.R., Hoffman, W.S., Dean, M.J., Armstrong, M.P., and Cadrin, S.X. 2014a. Spawning site fidelity by
Atlantic cod (Gadus morhua) in the Gulf of Maine: implications for population structure and rebuilding. ICES
Journal of Marine Science, 71(6): 1356–1365.
Zemeckis, D.R., M.J. Dean, and S.X. Cadrin. 2014b. Spawning dynamics and associated management
implications for Atlantic cod. North American Journal of Fisheries Management 34: 424–442.
