
ENDANGERED SPECIES RESEARCH
Endang Spec Res
INTRODUCTION
Within the Caribbean, centuries of commercial
exploitation have resulted in reduction or extirpation
of many marine turtle rookeries (Groombridge 1982,
Groombridge & Luxmoore 1989, Jackson 1997, Mc-
Clenachan et al. 2006). The Cayman Islands epitomize
this history of Caribbean marine turtle exploitation.
Despite their small size, the islands once supported
one of the largest ever green turtle Chelonia mydas
rookeries (Groombridge 1982), as well as abundant
nesting by loggerhead Caretta caretta and hawksbill
Eretmochelys imbricata turtles (Lewis 1940). It is
believed that every summer, marine turtles migrated to
the isolated islands to nest, leading to reports that ves-
sels, which have lost their latitude in hazy weather,
have steered entirely by the noise which these crea-
tures make in swimming to attain the Caymana isles
(Long 1774, cited in Lewis 1940 p. 57). By the early
1800s, massive exploitation caused a huge decline in
the Cayman Islands green turtle rookery (Lewis 1940),
and by the 20th century, rookeries here were consid-
ered extinct (Groombridge 1982). However, recent sur-
veys have revealed that nesting by green and logger-
head turtles persists at low levels (Wood & Wood 1994,
Aiken et al. 2001, Bell et al. in press). Additionally,
© Inter-Research 2006 · www.int-res.com*Email: [email protected]g
Satellite tracking highlights the need for
international cooperation in marine turtle
management
Janice M. Blumenthal
1, 2,
*
, Joni L. Solomon
1
, Catherine D. Bell
2
, Timothy J. Austin
1
,
Gina Ebanks-Petrie
1
, Michael S. Coyne
3
, Annette C. Broderick
2
, Brendan J. Godley
2
1
Department of Environment, PO Box 486, Grand Cayman KY1–1106, Cayman Islands
2
Marine Turtle Research Group, Centre for Ecology and Conservation, University of Exeter, Cornwall Campus,
Penryn TR10 9EZ, UK
3
Marine Geospatial Ecology Lab, Nicholas School of the Environment and Earth Sciences, Duke University, A321 LSRC,
PO Box 90328, Durham, North Carolina 27708, USA
ABSTRACT: We present detailed results of a satellite tracking project following 10 adult female tur-
tles from the Cayman Islands, thought to have once been one of the world’s largest rookeries. By
tracking the movements of 7 green turtles Chelonia mydas and 3 loggerhead turtles Caretta caretta
from now critically reduced rookeries we defined key habitats for internesting movement, migration,
and foraging in a range of Caribbean jurisdictions. Turtles tracked from the Cayman Islands traveled
to foraging grounds in Belize, Guatemala, Honduras, Mexico, Nicaragua and the USA. This range
encompasses a >2000 km stretch of Caribbean coastline and the Florida Keys, highlighting the need
for international cooperation in identifying and mitigating foraging ground threats. For one of the
green turtles, foraging site fidelity was elucidated over the course of two reproductive seasons, and
oceanic internesting intervals/post-nesting oceanic circles were defined for the first time in Atlantic
loggerhead turtles. In addition to fundamental and applied insights into the biology of the 2 species,
this research elucidates geographic scale for potential ecological effects of past decimation of rook-
eries in the Cayman Islands and highlights the effectiveness of community efforts in support of
conservation research.
KEY WORDS: Satellite tracking · Spatial ecology · Marine turtle · Chelonia mydas · Caretta caretta
Resale or republication not permitted without written consent of the publisher
Vol. 2: 51–61, 2006
Previously ESR 7: 1–11, 2006
Printed December 2006
Published online November 1, 2006

Endang Spec Res 2: 51–61, 2006
nesting by green turtles released from the Cayman
Turtle Farm as hatchlings or yearlings has been docu-
mented (Bell et al. 2005).
Green turtle rookeries in the Cayman Islands have
been reduced from a pre-exploitation estimate of 6.5
million adults (Jackson 1997), to near extirpation (less
than 26 nesting females of each species; Bell et al. in
press). Conservation of these critically reduced rook-
eries necessitates delineation of critical habitat and
identification of threats. Indeed, research on such
rookeries has recently been called for as part of a
regional review (McClenachan et al. 2006). However,
while nesting beaches in the Cayman Islands are com-
prehensively monitored (Bell et al. in press), marine
turtles spend most of their lives at sea. Over the past
few decades, application of flipper tags has been a
widely-utilized methodology for studying migration
and demographic parameters. However, this method is
not ideally suited to monitoring migration for small
rookeries, as the likelihood of tag recovery in such sit-
uations is extremely small. Additionally, tag recovery
locations may be biased toward areas with active
marine turtle fisheries, and it may be difficult to deter-
mine whether tags were recovered from final destina-
tions or locations en route (Godley et al. 2003,
Schroeder et al. 2003).
More recently, molecular genetic methods have
demonstrated that many foraging aggregations sup-
port turtles from multiple rookeries, and these methods
have proven to be of great utility in linking aggrega-
tions with their nesting beach origins (loggerhead tur-
tles: e.g. Engstrom et al. 2002, Bass et al. 2004, Roberts
et al. 2005, Maffucci et al. 2006, Reece et al. 2006;
green turtles: e.g. Lahanas et al. 1998, Bass & Witzell
2000, Luke et al. 2004). However, with the notable
exception of a green turtle foraging ground in
Nicaragua (Bass et al. 1998), adult foraging grounds in
the Caribbean are largely unsurveyed (Bowen 2003),
and if such surveys were undertaken, it
is unlikely that contributions from the
smallest rookeries would be detected.
Therefore, for these rookeries, satellite
tracking represents the only viable
technique for locating adult foraging
grounds.
Migrations of mature marine turtles
typically span hundreds or thousands of
kilometers (reviewed by Plotkin 2003).
Reproductively valuable adults are often
harvested and incidentally captured
during migration (Witherington 2003,
Seminoff 2004 Chelonia mydas. In:
IUCN 2006 IUCN Red List of Threatened
Species. Available at: www.redlist.org),
necessitating documentation of threats
on migration routes and foraging grounds (Morreale et
al. 1996, Lutcavage et al. 1997, Schroeder et al. 2003,
James et al. 2005). Satellite tracking facilitates rapid
identification of critical habitat (e.g. Horrocks et al. 2001,
James et al. 2005, Troëng et al. 2005), thus representing
a valuable tool for elucidating management require-
ments. In the case of small threatened rookeries the
methodology is particularly useful, as with moderate
capital investment detailed information can be gathered
very quickly.
By tracking wild green and loggerhead turtles, and a
green turtle reared in captivity, we set out to rapidly
delineate habitats (nesting beaches, internesting areas
migratory routes, and foraging grounds) utilized by
turtles originating from the Cayman Islands. In this
way, we aimed to highlight priorities for conservation.
MATERIALS AND METHODS
Study area. The Cayman Islands are located south of
Cuba in the Caribbean Sea (Fig. 1. Grand Cayman,
19.3° N, 81.4° W; Little Cayman, 19.7°N, 81.1° W; Cay-
man Brac, 19.7° N, 79.9° W). The 3 low-lying oceanic
islands are exposed carbonate peaks on the Cayman
Ridge with near-vertical slopes dropping to depths in
excess of 2000 m on all sides (Roberts 1994). The
islands provide approximately 55 km of shoreline suit-
able for marine turtle nesting (Aiken et al. 2001).
Platform Terminal Transmitter deployment. We
attached Platform Terminal Transmitters (PTTs) to 7
post-nesting green turtles (G1 to G7) and 3 post-nest-
ing loggerhead turtles (L1 to L3) from June to August
2003 (3 transmitters deployed), 2004 (5 transmitters
deployed) and 2005 (3 transmitters deployed). All log-
gerhead turtles and 6 of the green turtles were tagged
in Grand Cayman, while 1 green turtle was tagged in
Cayman Brac (Fig. 1). G1 was tracked for 2 reproduc-
52
Fig. 1. Cayman Islands, showing location of nesting sites for loggerhead tur-
tles (L1 to 3: filled circles) and green turtles (G1 to G7: open circles). 1000 m
contours are indicated

Blumenthal et al.: Satellite tracking of Cayman turtles
tive migrations; originally in 2003 (G1a), and again in
2004 after a 1 yr remigration interval (G1b). The turtle
was identified via inconel flipper tags applied the pre-
vious year and, as the first PTT had detached, we
deployed a new unit in order to track the turtle for a
second season. One of the green turtles (G4) had been
released from the Cayman Turtle Farm as a yearling in
1988, marked with a ‘living tag’ (implantation of a
4 mm disk of lightly colored plastron (lower shell) into
the darker carapace, with location of the tag coding for
year class) (Bell et al. 2005). At the time of transmitter
deployment in 2005, this turtle nested on Seven Mile
Beach, Grand Cayman (Cayman Islands Department
of Environment unpubl. data).
For a summary of deployment information, biomet-
rics, and satellite transmitter performance for all indi-
viduals see Table 1. Sirtrack KiwiSat 101 PTTs were
used on all individuals with the exception of 1 Sea
Mammal Research Unit (SMRU) Satellite Relay Data
Logger tag, which was deployed on L3. To attach the
units, a portable wooden corral was erected around
each turtle following nesting. The carapace of each
turtle was prepared by scrubbing to remove epibionts,
sanding lightly, and cleaning with acetone, and a PTT
was attached with 2-part epoxy (based on the methods
of Godley et al. 2003).
Data filtering. For all transmitters, data were down-
loaded from the ARGOS satellite system and analyzed
via a dedicated program known as the Satellite Track-
ing and Analysis Tool (STAT: Coyne & Godley 2005).
Location accuracy fell into 6 categories (location class
(LC) 3, 2, 1, 0, A, and B). The most accurate positions
(LC 3, 2, 1, A; cf. Hays et al. 2001) and biologically real-
istic speeds (<5 km h
–1
; cf. Luschi et al. 1998) were
used to reconstruct routes and calculate distances
traveled, although for loggerheads during migration
we also included LC B subject to a speed filter in cases
where fewer locations were available. Bathymetric
data were sampled from the General Bathymetric
Chart of the Oceans GEBCO 1-Minute Global Bathy-
metry Grid (www.bodc.ac.uk/projects/international/
gebco/gebco_digital_atlas).
RESULTS
Longevity and tracking
Green turtles were tracked for an extended period
from release to last transmission (mean ± SD 164 d ±
71, range 67 to 281, n = 8). In contrast, loggerhead
turtles were tracked for a consistently longer period
(mean ± SD 685 d ± 311, range 379 to 1000, n = 3).
Foraging destination was ascertained for all animals,
defined as >30 d at a fixed locale (<200 km in
diameter).
Green turtles
Movements between satellite transmitter deploy-
ment and the beginning of directed migration were
documented for green turtles (time in internesting
habitat, mean ± SD 17 d ± 14, range 0 to 34, n = 8). Prior
to commencing migrations to foraging habitat, the
majority of green turtles remained in the vicinity of the
nesting beach. However, 1 green turtle (G6) circled
Grand Cayman (Fig. 2a), and another (G7: the only
individual tagged in Cayman Brac) moved to the vicin-
ity of Grand Cayman (maximum distance from nesting
53
Turtle CCL PTT Tag Displacement Distance Migration Countries Foraging
(cm) deployment duration (km) traveled duration transited destination
date (d) (km) (d) (n)
G1a 104.0 2003-07-27 136 855 874 12 4 Guatemala
G1b 105.5 2004-08-28 67 856 1023 20 5 Guatemala
G2 107.0 2003-08-22 281 691 712 11 4 Belize & Mexico
G3 109.0 2004-08-19 144 768 1265 31 3 Mexico
G4 110.5 2005-07-24 112 745 991 18 3 Belize
G5 107.0 2005-08-28 88 632 466 19 3 Florida Keys
G6 110.0 2005-08-19 214 623 812 19 3 Mexico
G7 110.0 2004-08-31 269 520 548 11 2 Honduras
L1 100.0 2003-07-28 1000 791 1159 49 3 Nicaragua
L2 105.0 2004-07-14 675 535 886 39 3 Nicaragua
L3 100.0 2004-06-23 379 485 1020 27 3 Nicaragua
Table 1. Chelonia mydas and Caretta caretta. Summary of biometric, PTT deployment, and migration information for all turtles.
Information includes curved carapace length (CCL), PTT deployment date, straight-line distance from nesting beach to foraging
ground (displacement), total distance traveled, duration of migration, number of countries passed through during migration, and
final foraging destination for all tracked turtles. Turtle G1 was tracked in 2003 (G1a) and 2004 (G1b)

Endang Spec Res 2: 51–61, 2006
beach 197 km, mean depth of locations 2940 m) before
returning to Cayman Brac (Fig. 2b). While re-nesting
was not confirmed by beach patrol staff, it is likely that
these local movements occurred over the course of
internesting intervals. G7 reached Grand Cayman 10 d
after leaving Cayman Brac, and returned to Cayman
Brac after an additional 10 d. As this period represents
a typical internesting interval for green turtles in the
Cayman Islands (Cayman Islands Department of
Environment, unpubl. data), G7 may have shifted
nesting sites, nesting in Grand Cayman before return-
ing to Cayman Brac. Similarly, G6 returned to the
vicinity of the nesting beach on 2 occasions (after 10
and 12 d, respectively), suggesting that movements
may also have occurred over the course of 2 inter-
nesting intervals.
After beginning directed migration, 6 of the 7
tracked green turtles oriented toward the Central
American or Mexican mainland, while 1 individual
(G5) oriented northward toward Cuba and the Florida
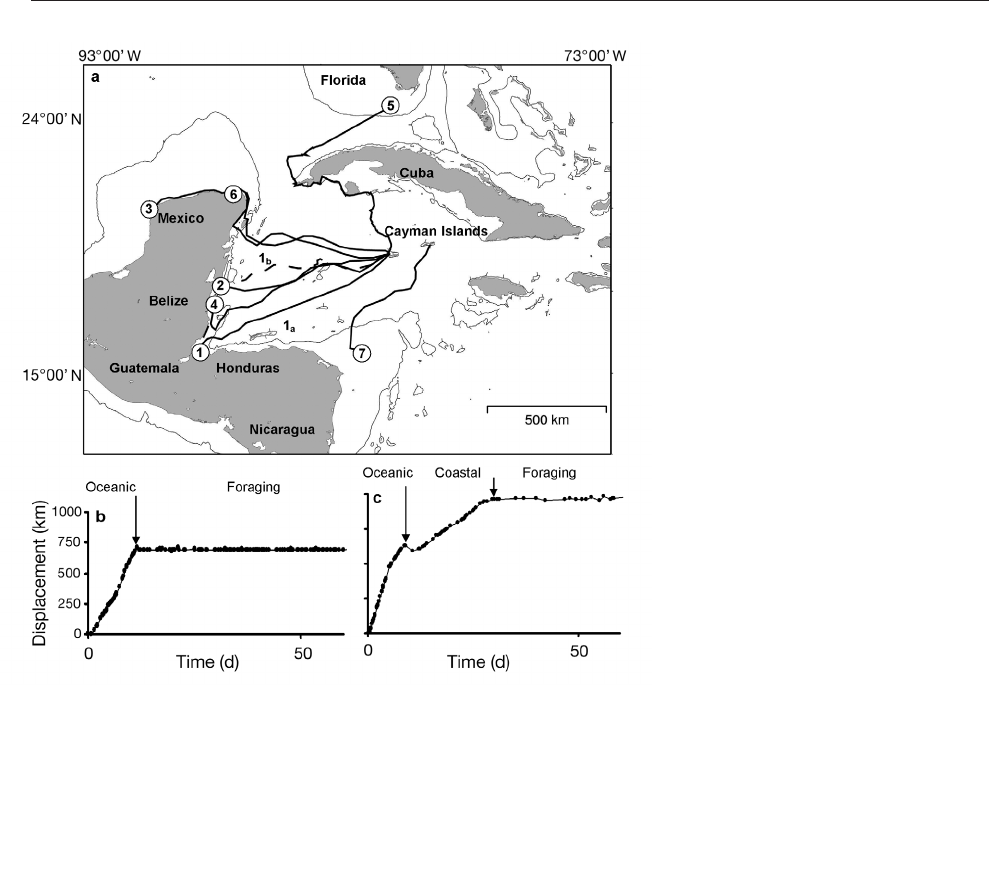
Keys (Fig. 3a). Two movement patterns were
observed: direct movement to foraging habitat (Fig.
3b, Individuals 2 and 7) and oceanic crossing fol-
lowed by coastal migration (Fig. 3c, Individuals 3, 4, 5
and 6). One individual (G1: tracked for 2 reproduc-
tive migrations) completed a direct oceanic migration
on the first occasion, and an indirect oceanic crossing
followed by coastal migration on the second. Duration
of migration for green turtles varied from 11 d
(entirely oceanic movement), to 31 d (9 d oceanic,
and 22 d in coastal waters). Of the 7 green turtles, 6
were tracked to shallow nearshore foraging areas,
while the green turtle tracked from Cayman Brac
(G7) took up residence in an offshore area (Gorda
Bank, Honduras).
Loggerhead turtles
Movements between satellite transmitter deploy-
ment and the beginning of directed migration were
documented for all loggerhead turtles (time in inter-
nesting habitat, mean ± SD 22 d ± 15, range 9 to 38,
n = 3). L1 remained near shore, while L2 displayed
entirely oceanic movements, circling Cayman Brac
and Little Cayman before returning to the vicinity of
Grand Cayman (Fig. 2c; maximum distance from the
nesting beach 135 km, mean depth of locations
2760 m). As duration of oceanic movement was 23 d,
and nesting does not appear to have occurred in
the other islands, this may represent a post-nesting
oceanic loop rather than an internesting interval. The
third individual (L3) initially circled offshore banks
(10 d), then remained in the vicinity of Grand Cayman
(15 d), and finally circled Cayman Brac and Little Cay-
man (10 d). While nesting was not confirmed, this
54
Fig. 2. Chelonia mydas and Caretta caretta. Post-nesting oceanic movements around the Cayman Islands for green and
loggerhead turtles (a) G6, (b) G7, (c) L2 and (d) L3. Other tracked turtles remained in the vicinity of the nesting beach prior
to beginning directed movements
Blumenthal et al.: Satellite tracking of Cayman turtles
movement pattern, and the seasonality of the move-
ments, suggests oceanic internesting intervals (Fig. 2d;
maximum distance from the nesting beach 211 km,
mean depth of locations 1900 m).
After beginning directed migration, all 3 loggerhead
turtles moved through oceanic waters toward the
mainland coast of Central America (Fig. 4). Logger-
head turtles utilized an indirect migratory strategy,
migrating along the coastal shelf to foraging destina-
tions. Two individuals (L1 and L2) encountered the
coastal shelf at the mouth of the Patuca River, Hon-
duras, and subsequently traveled east and south along
the shelf (in waters of <30 m). L2 initially took up resi-
dence near the Miskito Bank, Nicaragua, and L1 pro-
ceeded south to the vicinity of the Corn Islands,
Nicaragua. For L3, the complete post-nesting migra-
tory route could not be reconstructed due to a period of
few transmissions during migration. However, forag-
ing destination was obtained for this individual on the
edge of the Nicaraguan shelf in close proximity to L2.
The 3 loggerhead turtles utilized
Nicaraguan foraging grounds through-
out extended tracking, with L2 under-
taking a 200 km habitat shift to the
southern Nicaraguan shelf after more
than 4 mo at her initial foraging site.
After a 1 yr remigration interval, L3
returned to the vicinity of the Cayman
Islands and is presumed to have nested.
However, as the PTT was functioning
intermittently, a detailed return migra-
tory route could not be plotted.
DISCUSSION
Within 3 yr, through deployment of
satellite transmitters on 10 turtles, we
gained significant insights into the
movement of 2 critically reduced rook-
eries of Caribbean marine turtles. Esti-
mates of rookery size in the Cayman
Islands by Bell et al. (in press) suggest
that approximately 10 to 20% of
female loggerhead turtles and 30 to
40% of female green turtles were
tagged and tracked to foraging destina-
tions. Satellite tracking results may
therefore be representative of previ-
ously unknown migratory patterns for
the rookeries, providing swift insight
into movement across areas under a
range of jurisdictions.
Local habitat utilization
While some Cayman Islands green and loggerhead
turtles remained within a few km of the nesting
beach, others ranged through oceanic waters up to
200 km away. In prior studies, green and loggerhead
turtles generally remained in shallow areas near
nesting beaches before beginning migrations (green
turtles: Liew & Chan 1992, Meylan 1995, Hays et al.
2002a; loggerhead turtles: Hays et al. 1991, Godley
et al. 2003), though it should be noted that rookeries
in the Cayman Islands are in close proximity to
oceanic waters. For loggerhead turtles, oceanic habi-
tat utilization during the internesting interval has
been documented only in Japan (Sakamoto et al.
1990); however, the highly plastic behavior of this
species is gradually being revealed,with an in-
creasing number of studies showing oceanic resi-
dence in post-nesting adults (Hatase et al. 2002,
Hawkes et al. 2006).
55
Fig. 3. Chelonia mydas. (a) Green turtle migration routes to foraging grounds
in Central America, Mexico, and the USA. G1 was tracked for 2 successive
nesting seasons, 2003 (1a, solid line) and 2004 (1b, dashed line). Routes were
constructed utilizing positions of location class 3, 2, 1, A, and speeds less than
5 km h
–1
. The 200 m contour is indicated. (b,c) Displacement plots indicating
representative (b) oceanic (G2) and (c) coastal (G3) movement patterns. Slope of
the line is indicative of speed

Endang Spec Res 2: 51–61, 2006
For green turtles, post-nesting oceanic
loops (following the final nesting of the
season, yet prior to directed migration)
have recently been documented in Tor-
tuguero (Troëng et al. 2005) and Taiwan
(Cheng 2000). Looping behavior could be
associated with oceanic foraging (Troëng
et al. 2005) or attempts to locate a new
foraging ground (Luschi et al. 1998).
Although turtles from the Cayman
Islands briefly encountered shallower
waters, it is unlikely that benthic foraging
occurred, as mean depth of locations
during oceanic movements was greater
than 2500 m.
Oceanic movements in turtles from the
Cayman Islands may constitute oceanic
internesting intervals, shifts in nesting
beach utilization, or post-nesting oceanic
loops. Historically in the Cayman Islands,
exploitation on and near the nesting
beach brought an immense rookery to the
brink of extinction. During the nesting
season, reproductive individuals are typi-
cally concentrated in a small area within
a single jurisdiction, increasing vulnera-
bility to exploitation (Meylan 1995). For
rookeries which underwent heavy ex-
ploitation on and near the nesting beach,
oceanic movements during the internest-
ing period may have been advantageous.
It has been suggested that some turtles
nesting in the Cayman Islands may repre-
sent recent recruits, following the near
extirpation of the historical rookery
(Wood & Wood 1994, Aiken et al. 2001). If
this is the case, these colonizing individuals might be
expected to possess low nesting site fidelity, which
could increase the frequency of behaviors such as
oceanic movements during the internesting interval or
shifting between island nesting sites.
Migration
For post-nesting migration, 2 movement patterns
were observed: direct movements from the nesting
beach to the foraging ground and indirect oceanic
crossing followed by extended migration along the
coast or coastal shelf. Coastal migrations may serve 2
purposes: (1) facilitating navigation and (2) reducing
cost of migration by permitting benthic foraging (God-
ley et al. 2002, Hays et al. 2002b). Therefore, turtles
may minimize the duration of open ocean crossing,
even at the expense of increasing total migratory dis-
tance (Cheng 2000, Godley et al. 2002, Hays et al.
2002b). Movement patterns do not appear to be fixed
for individuals: the green turtle (G1) tracked for 2 con-
secutive reproductive migrations completed a direct
oceanic migration on one occasion and a coastal migra-
tion following an indirect ocean crossing on the other.
After both reproductive migrations, G1 maintained
fidelity to the same foraging site, suggesting orienta-
tion toward a distinct and fixed foraging ground rather
than selection of suitable habitat from year to year. In a
previous study, a green turtle satellite tracked through
successive breeding seasons did not return to her ini-
tial foraging site during the tracking period (Cheng
2000), although similar fidelity has been suggested in
adult female turtles in Australia via flipper tag returns
(Limpus et al. 1992, Limpus & Limpus 2001). In com-
parison to other turtles, the captive-bred green turtle
tracked in this study displayed no apparent anomalous
movements.
56
Fig. 4. Caretta caretta. (a) Loggerhead turtle migration routes to foraging grounds
in Central America. Routes were constructed utilizing positions of location class 3,
2, 1, A, B and speeds less than 5 km h
–1
. L2 initially foraged on the northern
Nicaraguan shelf (2
a
) before migrating 200 km south to forage on the southern
shelf (2
b
). The 200 m contour is indicated. (b) Displacement plot indicating re-
presentative indirect movement pattern of L1. (c) Combined displacement plots
for all loggerhead turtles, showing habitat shift from a northerly to a southerly
foraging site for L2

Blumenthal et al.: Satellite tracking of Cayman turtles
Lessons for regional conservation
Migration routes of turtles from the Cayman Islands
exemplify the problems of managing threatened
marine turtle rookeries, as turtles from a small area
dispersed widely through a wide range of habitats and
came under the jurisdiction of many nations. Green
turtles traveled to foraging grounds in Belize,
Guatemala, Honduras, Mexico, and the USA, with
their range encompassing a >2000 km stretch of
Caribbean coastline and the Florida Keys. This dis-
persion highlights the importance of broad and collab-
orative marine turtle management. In contrast, all 3
loggerhead turtles were tracked to foraging habitats in
Nicaragua, underscoring the necessity of identifying
key habitats and targeting action.
Anthropogenic impacts vary greatly with habitat and
jurisdiction: longline fisheries may threaten marine
turtles during oceanic movements, while directed take
and incidental capture in shrimp trawls and gill nets
are critical threats in the neritic zone (Lutcavage et al.
1997). For rookeries in the Cayman Islands, mortality
experienced on coastal migratory routes and foraging
grounds may represent a greater danger than, for
example, longlining, which generally occurs in oceanic
waters (Lewison et al. 2004). In the Gulf of Mexico and
the Caribbean Sea, shrimp trawls present a significant
threat (Groombridge & Luxmoore 1989, NRC 1990,
Henwood et al. 1992). Estimates based on 1987 shrimp
landings (Henwood et. al 1992) suggest that at the time
over 48000 turtles might have been incidentally cap-
tured in Mexican shrimp trawls each year, while over
15000 turtles might have been captured annually in
Central America (Henwood et al. 1992) (Table 2).
Accuracy of these estimates, species composition of
captured turtles, and current magnitude of this threat
are unknown, though these data are urgently required
to inform regional management.
In addition to experiencing a diversity of threats
during the course of reproductive migrations, green
and loggerhead turtles from rookeries in the Cayman
Islands are subject to many national and international
policies and legal instruments. Belize, Guatemala,
Honduras, Mexico, Nicaragua, and the USA are signa-
tories to the Convention on International Trade in
Endangered Species of Wild Fauna and Flora (CITES,
Chacón 2002), which prohibits international trade in
marine turtle products but does not regulate domestic
trade or habitat protection (Wold 2002). Other interna-
tional agreements — such as the Convention on the
Conservation of Migratory Species of Wild Animals
(CMS), the SPAW (Specially Protected Areas and
Wildlife) Protocol to the Cartagena Convention, and
the Inter-American Convention for the Protection and
Conservation of Sea Turtles (IAC) — have the potential
to effect international cooperation in marine turtle
management, but have not yet been consistently
adopted across the Caribbean region (Table 3). Addi-
tionally, the nature of national legislation and the
effectiveness of its enforcement varies greatly among
jurisdictions. Illegal turtle products are commonly mar-
keted in Belize, Guatemala, Honduras, and Mexico
(Chacón 2002, Fleming 2001) and in Nicaragua, which
appears to be key foraging habitat, it has been esti-
mated that a minimum of 10 000 to 11 000 green turtles
are taken annually, loggerhead turtles are taken inci-
dentally (Lagueux 1998).
As only the Cayman Islands are party to CMS, and
other national and international policies and instru-
ments are inconsistently applied, a thorough assess-
ment of the uptake and effectiveness of legislation is
needed to prioritize regional action. For green turtles
from the Cayman Islands, our results indicate that prio-
rities are in Central America and Mexico, while for
loggerhead turtles Honduras and Nicaragua appear to
be priority nations.
57
Longlines Shrimp trawls Incidental capture Directed take
Impact Species Impact Species Impact Species Impact Species
Cayman – – – – <5 Cc, Cm <5 Cc, Cm
Belize Un – 153
b
– Un – 500–800
d
Cc, Cm, Ei
Guatemala Un – 636
b
–Un– Un –
Honduras Un – 2899
b
–Un– Un –
Mexico Un – 48779
b
–Un– Un –
Nicaragua Un – 610
b
– Un – 10 000–11 000
e
Cm
USA 7891
a
Cc 5000–50 000
c
Cc 500–5000
c
Cc 5–50
c
Cc
Sources:
a
NOAA Fisheries (2001);
b
Henwood et al (1992);
c
NRC (1990);
d
Smith et al. (1992);
e
Lagueux (1998)
Table 2. Estimated annual marine turtle captures in jurisdictions utilized by mature green and loggerhead turtles from the
Cayman Islands. Nesting: Cayman Islands; Foraging: USA, Mexico, Belize, Guatemala, Honduras, and Nicaragua. Un: number
of marine turtle captures not quantified; Cc: loggerhead (Caretta caretta); Cm: green (Chelonia mydas); Ei: hawksbill
(Eretmochelys imbricata). –: threat does not occur

Endang Spec Res 2: 51–61, 2006
Ecological impacts
Although historical harvesting in the Cayman
Islands took place within the confines of a tiny geo-
graphic area and a single life-history stage, congrega-
tion of reproductive individuals allowed rookeries to
be decimated within a few decades (Lewis 1940). As
this ecological extinction occurred well before the
advent of scientific studies of the marine environment,
consequences for seagrass beds and other Caribbean
marine habitats are difficult to assess. However, histor-
ically, abundant marine turtles undoubtedly played a
major role in Caribbean marine ecosystems, acting as
grazers and consumers (Bjorndal & Jackson 2003,
Moran & Bjorndal 2005) and it is therefore presumed
that their loss had a drastic impact on the balance of
such ecosystems (McClenachan et al. 2006).
Additionally, the near extirpation of nesting turtles
in the Cayman Islands may have had demographic
consequences for other Caribbean rookeries: a den-
sity-dependent effect on foraging grounds (Bjorndal
et al. 2000) may have facilitated compensatory popu-
lation growth in extant Caribbean green turtle rook-
eries, such as Tortuguero, which has experienced a
positive population trend (Troëng & Rankin 2005).
Patterns of dispersion of remnant rookeries must be
interpreted with caution, but by tracking surviving
members of what may have been one of the largest
ever green turtle rookeries (Groombridge 1982) and a
significant Caribbean loggerhead rookery (Lewis
1940), we may be able to glimpse historical range and
trace impacts of ecological extinction through wide-
spread ecosystems.
In addition to impacting foraging grounds in a range
of Caribbean jurisdictions, the extirpation of rookeries
from the Cayman Islands is likely to have profoundly
impacted local ecosystems. As turtles mediate signifi-
cant energy flow from nutrient-rich foraging grounds
to nutrient-poor nesting beaches (Bouchard & Bjorndal
2000), abundant nesting by green and loggerhead tur-
tles is likely to have affected the growth of native
beach vegetation and the nutrient content of lagoonal
waters off nesting beaches. Therefore, near extinction
of these rookeries will have brought about significant
shifts in ecosystem dynamics prior to modern surveys
of reef environments.
Insights into local conservation: community-
sponsored conservation efforts
While rookeries in the Cayman Islands are now
critically small (Bell et al. in press), resident juvenile
hawksbill and green turtles are often sighted around
all 3 islands. Due to this apparent ‘abundance’ of
turtles, management requirements for rookeries can
be difficult to convey (J. M. Blumenthal pers. obs.).
Reproductively valuable adult and sub-adult green
and loggerhead turtles are targeted in a legal fish-
ery, and illegal take of eggs and nesting females
continues in the Cayman Islands (Bell et al. 2006).
Satellite tracking has demonstrated the migratory
nature of marine turtle rookeries (versus the year-
round presence of juveniles), and has facilitated
scientific and community discussion of the re-
productive value of mature turtles. This concept has
great applicability throughout the Caribbean region,
as management legislation is often focused on mi-
nimum rather than maximum size limits, aimed at
protecting juveniles rather than adults (Richardson
et al. 2006).
As charismatic species, marine turtles provide an
ideal flagship for introducing communities to conser-
vation concepts. Funds for satellite tracking turtles
58
CITES CMS Cartagena SPAW IAC CBD
Party Party Signed Ratified Signed Ratified Signed Ratified Signed Ratified
Cayman Is. Yes Yes Yes Yes No No No No Yes Yes
Belize Yes No – Yes No No Yes Yes Yes Yes
Guatemala Yes No Yes Yes Yes No Yes Yes Yes Yes
Honduras Yes No Yes No No No Yes Yes Yes Yes
Mexico Yes No Yes Yes Yes No Yes Yes Yes Yes
Nicaragua Yes No Yes No No No Yes No Yes Yes
USA Yes No Yes Yes Yes Yes Yes Yes Yes No
Table 3. Participation in Multilateral Environmental Agreements (MEAs) in jurisdictions utilized by adult female green and
loggerhead turtles from the Cayman Islands. Nesting: Cayman Islands; foraging: USA, Mexico, Belize, Guatemala, Honduras,
Nicaragua. MEA abbreviations: CITES: Convention on International Trade in Endangered Species of Wild Fauna and Flora;
CMS: Convention on the Conservation of Migratory Species of Wild Animals; Cartagena: Convention for the Protection and
Development of the Marine Environment of the Wider Caribbean Region (Cartagena Convention); SPAW: protocol concerning
Specially Protected Areas and Wildlife (SPAW Protocol to the Cartagena Convention); IAC: Inter-American Convention for
the Protection and Conservation of Sea Turtles; CBD: Convention on Biological Diversity

Blumenthal et al.: Satellite tracking of Cayman turtles
from the Cayman Islands were largely raised by school
fundraisers and from community sponsors, and migra-
tory paths were made available to the public in near
real time on the SEATURTLE.ORG satellite tracking
website (www.seaturtle.org/tracking). In the past 3 yr,
the tracking website as a whole has received more
than 3.5 million visits from over 155 countries. With this
participatory approach, we aimed to focus awareness
on the need for international cooperation in marine
turtle management.
Overall, satellite tracking marine turtles from the
Cayman Islands across international boundaries pro-
vided an accessible introduction to issues of interna-
tional law surrounding migratory species and offered
an unparalleled opportunity to increase awareness
among the public and policy makers. This research has
enabled us to develop and communicate an under-
standing of management requirements for threatened
marine turtle rookeries, while providing a case study in
the difficulties of managing highly-migratory endan-
gered species.
Acknowledgements. Graphics were produced using the
Maptool program (a product of SEATURTLE.ORG: www.
seaturtle.org/maptool). All tracks were made available to
the public in near real time on the SEATURTLE.ORG track-
ing website: www.seaturtle.org/tracking. We thank Cayman
Islands volunteers and sponsors, particularly M. Orr, E.
Blanco, G. Oberholtzer, G. Kwong, J. Porter, M. Fowlds, the
Olde family, the Richardson family, St. Ignatius High
School, Ocean Frontiers Ltd, Jacques Scott Ltd, DiveTech
Kids Camp Outreach Program, the Brakka Trakkas, the
Ritz-Carlton Grand Cayman’s Ambassadors of the Environ-
ment program, and Department of Environment research,
enforcement, and operations staff. Work in the Cayman
Islands was supported by the National Fish and Wildlife
Foundation (NFWF), and work in the UK was supported by
the Darwin Initiative, European Social Fund, Overseas Ter-
ritories Environment Programme, Turtles in the UK Over-
seas Territories (TUKOT) and the National Environment
Research Council (NERC). J.B. is supported by a University
of Exeter Postgraduate Studentship. The manuscript was
improved by the input of 3 anonymous reviewers.
LITERATURE CITED
Aiken JJ, Godley BJ, Broderick AC, Austin T, Ebanks-Petrie
G, Hays GC (2001) Two hundred years after a commercial
marine turtle fishery: the current status of marine turtles
nesting in the Cayman Islands. Oryx 35:145–151
Bass AL, Witzell WN (2000) Demographic composition of
immature green turtles (Chelonia mydas) from the east
central Florida coast: evidence from mtDNA markers.
Herpetologica 56 (3):357–67
Bass AL, Lagueux CJ, Bowen BW (1998) Origin of green tur-
tles, Chelonia mydas, at ‘Sleeping Rocks’ off the northeast
coast of Nicaragua. Copeia 1998 (4):1064–1069
Bass AL, Epperly SP, Braun-McNeil J (2004) Multi-year
analysis of stock composition of a loggerhead turtle
(Caretta caretta) foraging habitat using maximum likeli-
hood and Bayesian methods. Conserv Genet 5:783–796
Bell CDL, Parsons J, Austin TJ, Broderick AC, Ebanks-Petrie
G, Godley BJ (2005) Some of them came home: the Cay-
man Turtle Farm headstarting project for the green turtle
Chelonia mydas. Oryx 39:137–148
Bell CD, Blumenthal JM, Austin TJ, Solomon JL, Ebanks-
Petrie G, Broderick AC, Godley BJ (2006) Traditional
Caymanian fishery may impede local marine turtle popu-
lation recovery. Endang Spec Res (in press)
Bell C, Solomon JL, Blumenthal JM, Austin TJ, Ebanks-Petrie
G, Broderick AC, Godley BJ (in press) Monitoring and
conservation of critically reduced marine turtle nesting
populations: lessons from the Cayman Islands. Anim
Conserv
Bjorndal KA, Jackson JBC (2003) Roles of sea turtles in
marine ecosystems: reconstructing the past. In: Lutz PL,
Musick JA, Wyneken J (eds) The biology of sea turtles,
Vol II. CRC Press, Boca Raton, FL, p 259–273
Bjorndal KA, Bolten AB, Chaloupka MY (2000) Green turtle
somatic growth model: evidence for density dependence.
Ecol Appl 10(1):269–282
Bouchard SS, Bjorndal KA (2000) Sea turtles as biological
transporters of nutrients and energy from marine to terres-
trial ecosystems. Ecology 81:2305–2313
Bowen BW (2003) What is a loggerhead turtle? The genetic
perspective. In: Bolten AB, Witherington B (eds) The biol-
ogy of loggerhead sea turtles. Smithsonian Institution
Press, Washington, DC, p 7–27
Chacón D (2002) Diagnóstico sobre el comercio de las tortu-
gas marinas y sus derivados en el istmo Centroamericano.
Red regional para la conservación de las tortugas marinas
en Centroamérica (RCA). San José
Cheng IJ (2000) Post-nesting migrations of green turtles
(Chelonia mydas) at Wan-An Island, Penghu Archipelago,
Taiwan. Mar Biol 137:747–754
Coyne MS, Godley BJ (2005) Satellite Tracking and Analysis
Tool (STAT): an integrated system for archiving, analyzing
and mapping animal tracking data. Mar Ecol Prog Ser
301:1–7
Engstrom TN, Meylan PA, Meylan AB (2002) Origin of juve-
nile loggerhead turtles (Caretta caretta) in a tropical
developmental habitat in Caribbean Panama. Anim Con-
serv 5:125–133
Fleming EH (2001) Swimming against the tide: recent
surveys of exploitation, trade and management of ma-
rine turtles in the northern Caribbean. TRAFFIC, Wash-
ington, DC
Godley BJ, Richardson S, Broderick AC, Coyne MS, Glen F,
Hays GC (2002) Long-term satellite telemetry of the
movements and habitat utilisation by green turtles in the
Mediterranean. Ecography 25:352–362
Godley BJ, Broderick AC, Glen F, Hays GC (2003) Post-nest-
ing movements and submergence patterns of loggerhead
marine turtles in the Mediterranean assessed by satellite
tracking. J Exp Mar Biol Ecol 287:119–134
Groombridge B (1982) The IUCN amphibia reptilia red data
book, Part I. IUCN, Gland, p 201–207
Groombridge B, Luxmoore R (1989) The green turtle and
hawksbill (Reptilia: Cheloniidae): world status, exploita-
tion and trade. CITES Secretariat, Lausanne
Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K,
Goto K, Arai N, Fujiwara T (2002) Size-related differences
in feeding habitat use of adult female loggerhead turtles
Caretta caretta around Japan determined by stable iso-
tope analyses and satellite telemetry. Mar Ecol Prog Ser
233:273–281
59

Endang Spec Res 2: 51–61, 2006
Hays GC, Webb PI, Hayes JP, Priede IG, French J (1991)
Satellite tracking of a loggerhead turtle (Caretta caretta)
in the Mediterranean. J Mar Biol Assoc UK 71:743–746
Hays GC, Akesson S, Godley BJ, Luschi P, Santidrian P (2001)
The implications of location accuracy for the interpretation
of satellite tracking data. Anim Behav 61:1035–1040
Hays GC, Glen F, Broderick AC, Godley BJ, Metcalfe JD
(2002a) Behavioural plasticity in a large marine herbivore:
contrasting patterns of depth utilisation between two
green turtle (Chelonia mydas) populations. Mar Biol 141:
985–990
Hays GC, Broderick AC, Godley BJ, Lovell P, Martin C,
McConnel BJ, Richardson S (2002b) Bi-phasal long-dis-
tance migration in green turtles. Anim Behav 64:895–898
Hawkes LA, Broderick AC, Coyne MS, Godfrey MS and
5 others (2006) Phenotypically linked dichotomy in sea
turtle foraging requires multiple conservation approaches.
Curr Biol 16:990–995
Henwood T, Stuntz W, Thompson N (1992) Evaluation of US
turtle protective measures under existing TED regula-
tions, including estimates of shrimp trawler related mor-
tality in the wider Caribbean. NOAA Tech Memo NMFS-
SEFSC-303, Silver Spring, MD
Horrocks JA, Vermeer LA, Krueger B, Coyne M, Schroeder
BA, Balazs GH (2001) Migration routes and destination
characteristics of post-nesting hawksbill turtles satellite-
tracked from Barbados, West Indies. Chelonian Conserv
Biol 4:107–114
Jackson JBC (1997) Reefs since Columbus. Coral Reefs
16:S23–S32
James MC, Ottensmeyer CA, Myers RA (2005) Identification
of high-use habitat and threats to leatherback sea turtles
in northern waters: new directions for conservation. Ecol
Lett 8:195–201
Lagueux CJ (1998) Marine turtle fishery of Caribbean
Nicaragua: human use patterns and harvest trends. PhD
dissertation, University of Florida, Gainesville, FL
Lahanas PN, Bjorndal KA, Bolten AB, Encalada SE, Miyamoto
MM, Valverde RA, Bowen BW (1998) Genetic composition
of a green turtle (Chelonia mydas) feeding ground popu-
lation: evidence for multiple origins. Mar Biol 130:345–352
Lewis CB (1940) The Cayman Islands and marine turtle. In:
Grant C (ed) The herpetology of the Cayman Islands. Bul-
letin of the Institute of Jamaica Science Series 2, Institute
of Jamaica, Kingston, p 56–65
Lewison RL, Freeman SA, Crowder LB (2004) Quantifying the
effects of fisheries on threatened species: the impact of
pelagic longlines on loggerhead and leatherback sea tur-
tles. Ecol Lett 7:221–231
Liew HC, Chan EH (1993) Biotelemetry of green turtles (Che-
lonia mydas) in Pulau Redang, Malaysia, during the
internesting period. In: Paolo M, Sandro F, Cristina C,
Remo B (eds) Biotelemetry XII: Proc 12th Int Symp
Biotelemetry, 31 Aug–5 Sep 1992. Ancona. Litograffia
Felici, Pisa, p 157–163
Limpus CJ, Limpus DJ (2001) The loggerhead turtle, Caretta
caretta, in Queensland: breeding migrations and fidelity
to a warm temperate feeding area. Chelonian Conserv
Biol 4 (1):142–153
Limpus CJ, Miller JD, Parmenter CJ, Reimer D, McLachlan N,
Webb R (1992) Migration of green (Chelonia mydas) and
loggerhead (Caretta caretta) turtles to and from Eastern
Australian Rookeries. Wildl Res 19:347–358
Long E (1774) History of Jamaica, or general survey of the
ancient and modern state of that island. George Metcalf,
Frank Cass & Co, London
Luke K, Horrocks JA, LeRoux RA, Dutton PH (2004) Origins of
green turtle (Chelonia mydas) feeding aggregations
around Barbados, West Indies. Mar Biol 144:799–805
Luschi P, Hays GC, Del Seppia C, Marsh R, Papi F (1998) The
navigational feats of green sea turtles migrating from
Ascension Island investigated by satellite telemetry. Proc
R Soc Lond Ser B 265:2279–2284
Lutcavage ME, Plotkin P, Witherington B, Lutz PL (1997)
Human impacts on sea turtle survival. In: Lutz PL, Musick
JA (eds) The biology of sea turtles. CRC Marine Science
Series. CRC Press, Boca Raton, FL, p 387–409
Maffucci F, Kooistra WHCF, Bentivegna F (2006) Natal origin
of loggerhead turtles, Caretta caretta, in the neritic habitat
off the Italian coasts, Central Mediterranean. Biol Conserv
127:183–189
McClenachan L, Jackson JBC, Newman MJH (2006) Conser-
vation implications of historic sea turtle nesting beach loss.
Front Ecol Environ 4 (6):290–296
Meylan AB (1995) Behavioral ecology of the west Caribbean
green turtle (Chelonia mydas) in the internesting habitat.
In: Bjorndal KA (ed) Biology and conservation of sea tur-
tles, revised edn. Smithsonian Institution Press, Washing-
ton, DC, p 67–80
Moran KL, Bjorndal KA (2005) Simulated green turtle grazing
affects structure and productivity of seagrass pastures.
Mar Ecol Prog Ser 305:235–247
Morreale SJ, Standora EA, Spotila JR, Paladino FV (1996)
Migration corridor for sea turtles. Nature 384:319–320
NOAA Fisheries (2001) Endangered Species Act section 7
consultation — reinitiation of consultation on the Atlantic
highly migratory species fishery management plan and its
associated fisheries. National Marine Fisheries Service,
Silver Spring, MD
NRC (National Research Council) (1990) Decline of the sea
turtles: causes and prevention. National Research Coun-
cil, Washington, DC
Plotkin P (2003) Adult migrations and habitat use. In: Lutz PL,
Musick JA, Wyneken J (eds) The biology of sea turtles, vol
II. CCR Press. Boca Raton, FL, p 225–241
Reece JS, Ehrhart LM, Parkinson CL (2006) Mixed stock
analysis of juvenile loggerhead turtles in the Indian River
Lagoon and implications for marine turtle conservation
planning. Conserv Genet 7:345–352
Richardson PB, Broderick AC, Campbell LM, Godley BJ,
Ranger S (2006) Marine turtle fisheries in the UK Overseas
Territories of the Caribbean: domestic legislation and the
requirements of multilateral agreements. J Int Wildl Law
Pol 9:223–246
Roberts H (1994) Reefs and lagoons of Grand Cayman. In:
Brunt MA, Davies JE (eds) The Cayman Islands: natural
history and biogeography, Kluwer Academic Publishers,
Dordrecht, p 75–104
Roberts MA, Anderson CJ, Stender B, Segars A, Whittaker
JD, Grady JM, Quattro JM (2005) Estimated contribution
of Atlantic coastal loggerhead turtle nesting populations
to offshore feeding aggregations. Conserv Genet
6:133–139
Sakamoto W, Uchida I, Naito Y, Kureha K, Tujimura M, Sato
K (1990) Deep diving behaviour of the Loggerhead turtle
near the frontal zone. Nippon Suisan Gakkaishi
56:1435–1443
Schroeder BA, Foley AM, Bagley DA (2003) Nesting patterns,
reproductive migrations, and adult foraging areas of log-
gerhead turtles. In: Bolten AB, Witherington BE (eds) Log-
gerhead sea turtles. Smithsonian Books, Washington, DC,
p 114–124
Smith GW, Eckert KL, Gibson JP (1992) WIDECAST sea turtle
recovery action plan for Belize. In: Eckert, KA (ed) CEP
60

Blumenthal et al.: Satellite tracking of Cayman turtles
Technical Report No 18. UNEP Caribbean Environment
Programme, Kingston
Troëng S, Rankin E (2005) Long-term conservation efforts
contribute to positive green turtle Chelonia mydas nesting
trend at Tortuguero, Costa Rica. Biol Conserv 121:
111–116
Troëng S, Evans DR, Harrison E, Lagueux CJ (2005) Migra-
tion of green turtles Chelonia mydas from Tortuguero
Costa Rica. Mar Biol 148:435–447
Witherington BE (2003) Biological conservation of logger-
heads: challenges and opportunities. In: Bolten AB, With-
erington BE (eds) Loggerhead sea turtles. Smithsonian
Books, Washington, DC, p 295–311
Wold C (2002) The status of sea turtles under international
environmental law and international environmental
agreements. J Int Wildl Law Pol 5:11–48
Wood FE, Wood JR (1994) Sea turtles of the Cayman Islands.
In: Brunt MA, Davies JE (eds) The Cayman Islands: nat-
ural history and biogeography. Kluwer Academic Publish-
ers, Dordrecht, p 229–236
61
Editorial responsibility: Helene Marsh,
Townsville, Queensland, Australia
Submitted: June 14, 2006; Accepted: September 23, 2006
Proofs received from author(s): October 15, 2006
