
1
Catalyst One*
Chemistry Analyzer
> OPERATOR’S GUIDE
2
Proprietary rights notice
Information in this document is subject to change without notice. Companies, names, and data used in examples
are ctitious unless otherwise noted. No part of this document may be reproduced or transmitted in any form or by
any means, electronic, mechanical, or otherwise, for any purpose, without the express written permission of IDEXX
Laboratories. IDEXX Laboratories may have patents or pending patent applications, trademarks, copyrights, or other
intellectual or industrial property rights covering this document or subject matter in this document. The furnishing
of this document does not give a license to these property rights except as expressly provided in any written license
agreement from IDEXX Laboratories.
© 2024 IDEXX Laboratories, Inc. All rights reserved. • 06-0001252-11
*IDEXX VetLab, Catalyst, Catalyst One, VetTrol, SmartLink, IDEXX InterLink, IDEXX SmartService, SNAP, and 4Dx are
trademarks or registered trademarks of IDEXX Laboratories, Inc. in the United States and/or other countries. All other
product and company names and logos are trademarks of their respective holders.
3
Contents
Preface ...................................................................................................................................................... 5
Safety Precautions ..................................................................................................................................................... 5
Performance Precaution........................................................................................................................................... 5
Care of the Analyzer .................................................................................................................................................. 5
International Symbol Descriptions ......................................................................................................................... 6
Other Symbols ............................................................................................................................................................ 7
Getting Started .......................................................................................................................................... 8
Introduction ................................................................................................................................................................. 8
Catalyst One Components ....................................................................................................................................... 9
Analyzer Status .........................................................................................................................................................10
Responding to an Alert............................................................................................................................................11
Installing the Catalyst One Analyzer .....................................................................................................................11
Catalyst One Analyzer Consumables ................................................................................................................... 12
Compatible Species .................................................................................................................................................13
Using the Catalyst One* Analyzer ............................................................................................................. 14
Analyzing Samples ..................................................................................................................................................14
Slide Handling ........................................................................................................................................................... 14
Diluting Samples ......................................................................................................................................................14
Viewing and Printing Test Results .......................................................................................................................16
Outside of Reportable Range Samples ...............................................................................................................16
Modifying the Settings on the Analyzer ................................................................................................... 18
Modifying the Sound Settings
‡
..............................................................................................................................18
Entering Standby Mode...........................................................................................................................................18
Exiting Standby Mode .............................................................................................................................................18
Sample Preparation and Storage ............................................................................................................. 19
Supported Sample Types for Catalyst* CLIPs and Slides ................................................................................ 19
Preparing Samples for Use on the Catalyst One Analyzer ............................................................................... 20
Proper Sample Cup Volume ...................................................................................................................................22
Sample Inspection After Centrifugation ..............................................................................................................22
Sample Storage ........................................................................................................................................................23
Quality Control ......................................................................................................................................... 24
Overview ....................................................................................................................................................................24
Quality Control Materials .......................................................................................................................................24
Quality Control CLIPs and Slides ...........................................................................................................................25
Preparing Control Fluid ...........................................................................................................................................26
Running Quality Control ..........................................................................................................................................27
4
Maintenance ............................................................................................................................................ 28
Overview ....................................................................................................................................................................28
Upgrading the Software .......................................................................................................................................... 28
Cleaning the Internal Components of the Analyzer ...........................................................................................28
Cleaning the Outside of the Analyzer
and the Sample Drawer .................................................................................................................................... 29
Emptying the Waste Drawer ..................................................................................................................................29
Appendices .............................................................................................................................................. 30
Chemistry Descriptions...........................................................................................................................................30
Medical Protocol Descriptions ..............................................................................................................................55
Differences in Results .............................................................................................................................................60
Technical Specications .........................................................................................................................................60
IDEXX Customer and Technical Support contact information........................................................................ 61
5
Preface
Safety Precautions
Note: If the equipment is used in a manner other than specied, the protection provided by the equipment
may be impaired.
The analyzer does not contain any user-serviceable components. DO NOT disassemble.
Line voltage for the Catalyst One AC power adapter is 100–240 V AC, 50–60 Hz. Be sure to plug all
equipment into properly grounded electrical outlets.
Use only the AC power adapter and AC power cable supplied.
Disconnect the power cable:
+ If the cable becomes frayed or otherwise damaged.
+ If anything is spilled onto the equipment.
+ If your equipment is exposed to excessive moisture.
+ If your equipment is dropped or the case has been damaged.
+ If you suspect that your analyzer needs service or repair.
+ Whenever you clean the case.
Performance Precaution
Do not use certain liquids, aerosols (such as canned air), solvents, ammonia, and other substances on or
near the analyzer which could inuence results.
Care of the Analyzer
It is recommended that you do not stack other equipment or containers on top of the analyzer.
Keep analyzer away from sources of heat or ames.
PROTECT your equipment from damp conditions, wet weather, or liquid spills.
Take care not to spill water or other liquids on the unit.
DO NOT use solvents, ink markers, sprays containing volatile liquids, or polish on the analyzer as it may
damage the outer case. Clean only with a mild soap and slightly moist cloth and only when the analyzer is
not in use.
Clean only with a mild soap and slightly moist cloth and only when the analyzer is not in use.

6
International Symbol Descriptions
International symbols are often used on packaging to provide a pictorial representation of particular
information related to the product (such as expiration date, temperature limitations, batch code, etc.). IDEXX
Laboratories has adopted the use of international symbols on our analyzers, product boxes, labels, inserts,
and manuals in an effort to provide our users with easy-to-read information.
Symbol Description Symbol
Description
Use by
A utiliser avant
Verwendbar bis
Usare entro
Usar antes de
使用期限
Temperature limitation
Température limite
Zulässiger Temperaturbereich
Temperatura limite
Limitación de temperatura
保 存 温 度( 下 限 )
Batch code (Lot)
Code de lot (Lot)
Chargenbezeichnung (Partie)
Codice del lotto (partita)
Código de lote (Lote)
ロット 番 号
Upper limit of temperature
Limite supérieure de température
Temperaturobergrenze
Limite superiore di temperatura
Limite superior de temperatura
保 存 温 度( 上 限 )
Serial number
Numéro de série
Seriennummer
Numero di serie
Número de serie
シリアル番号
Consult instructions for use
Consulter la notice d’utilisation
Gebrauchsanweisung beachten
Consultare le istruzioni per l’uso
Consultar las instrucciones de uso
取扱説明書をご参照ください。
Catalog number
Numéro catalogue
Bestellnummer
Numero di catalogo
Número de catálogo
製品番号
Keep away from sunlight
Conserver à l’abri de la lumière
Vor direkter Sonneneinstrahlung
schützen
Mantener alejado de la luz solar
Tenere lontano dalla luce diretta del sole
遮 光してくだ さ い 。
Authorized Representative in the
European Community
Représentant agréé pour la C.E.E.
Autorisierte EG-Vertretung
Rappresentante autorizzato nella
Comunitá Europea
Representante autorizado en la
Comunidad Europea
EC内の正規販売代理店
WEEE Directive 2002/96/EC
Directive 2002/96/CE (DEEE)
WEEE-Richtlinie 2002/96/EG
Directiva 2002/96/CE RAEE
Direttiva RAEE 2002/96/CE
廃電気電子機器指令(WEEE Directive
2002/96/EC)
Manufacturer
Fabricant
Hersteller
Ditta produttrice
Fabricante
製造元
Biological risks
Risques biologiques
Biogefährlich
Rischi biologici
Riesgos biológicos
生物学的リスク
Caution, consult accompanying
documents
Attention, consulter les documents
joints
Achtung, Begleitdokumente
beachten
Attenzione, consultare la
documentazione allegata
Precaución, consultar la
documentación adjunta
注意、添付文書をご参照ください。
2
Do not reuse
Usage unique
Nicht wiederverwenden
No reutilizarw
Non riutilizzare
再 利用しないでください。
Preface

7
Symbol Description Symbol
Description
Caution, hot surface
Attention, surface très chaude
Precaución, supercie caliente
Vorsicht, heiße Oberäche
Attenzione, supercie rovente
高温注意
Electrostatic-sensitive device
Appareil sensible aux charges
éléctrostatiques
Dispositivo sensible a descargas
electrostáticas
Gerät ist sensibel auf elektrostatische
Ladung
Dispositivo sensibile alle scariche
elettrostatiche
静電気の影響を受ける装置
Keep dry
Conserver dans un endroit sec
Mantener seco
Vor Nässe schützen
Tenere al riparo dall’umidità
濡らさないこと。
Fragile
Fragile
Frágil
Zerbrechlich
Fragile
取扱注意
This side up
Haut
Este lado hacia arriba
Diese Seite nach oben
Alto
この面を上にする。
Date of manufacture
Date de production
Fecha de producción
Herstelldatum
Data di produzione
製造年月日:
Do not freeze
Other Symbols
Symbol Description Symbol Description
USB symbol Ethernet/network symbol
Preface

8
Getting Started
Introduction
Welcome to IDEXX’s next-generation chemistry analyzer—the Catalyst One* Chemistry Analyzer.
The Catalyst One analyzer’s exible test menu allows you to monitor the health status of specic organs,
recheck values over time, customize proles by adding single tests to CLIPs. You can even run up to 25
tests
on a single sample (see a complete list of the individual slides and CLIPs available).
The Catalyst One analyzer is for veterinary use only.
IDEXX VetLab* Station Connectivity
The Catalyst One analyzer is part of the IDEXX VetLab* suite of analyzers, all of which connect to the IDEXX
VetLab Station (IDEXX’s laboratory information management system). Connecting multiple analyzers to the
IDEXX VetLab Station helps you attain a comprehensive picture of your patient’s health, with the ability to
view test results from multiple analyzers on a single report, determine disease progression with parameter-
trending capabilities, and more.
By connecting the Catalyst One analyzer to the IDEXX VetLab Station, you can:
+ Automatically review patients’ prior results on every printout for easy comparison.
+ Improve client communications with illustrated diagnostic or treatment progress printouts.
+ Link to expert descriptions and common causes of abnormal values.
+ Print information to help explain the signicance of results to your clients.
+ Allow new staff to train independently.
+ Learn proper protocols and tips for best techniques.
Proprietary Slide Technologies
Proprietary technologies in Catalyst* slides minimize interfering substances:
+ IDEXX dry-slide technology uses multiple technologies that minimize interfering substances as the
sample moves from the top to bottom layer, where it is analyzed.
+ Scavenging and/or spreading layers lter interferants from other blood chemistry components to
ensure sample quality.
+ An integrated wash process is used with specic slides to remove debris from the sample, maximizing
sensitivity and the accuracy of results.

9
Catalyst One Components
Front of the Analyzer
Side door
Sample drawer
Waste drawer
Status LED
Start/power
button
Lock light
Inside of the Sample Drawer
Note: This picture depicts where the sample cup and whole blood separator should be placed in the sample
drawer. Do not load a whole blood separator AND a sample cup for a single run.
Sample
cup
Slides/CLIPs Phenobarbital
(PHBR) reagent
consumable/automated
dilution cups
Whole blood
separator
Pipette tips
Other reagent
consumables
Getting Started

10
Side of the Analyzer
Side door
Carousel cover
(shown closed)
Lever to raise
carousel cover
Back of the Analyzer
Ethernet port
Power port
Analyzer Status
The light-emitting diode (LED) indicator on the front panel of the Catalyst One analyzer indicates the
analyzer’s status.
Note: You can also view the analyzer status by viewing its icon on the IDEXX VetLab Station Home screen.
LED Color Description
Green (steady) READY; analyzer is ready to process samples or perform
maintenance tasks
Green (pulse) STANDBY MODE
Yellow (steady) IN PROCESS; analyzer is processing a sample or performing
another activity
Yellow (pulse) Analyzer is waiting for the user to begin processing a sample after
receiving the patient information from the IDEXX VetLab Station
Red (ashing) ERROR; an error has occurred; review error or alert messages on
the IDEXX VetLab Station
Getting Started
11
Responding to an Alert
When the analyzer experiences a problem, an alert message appears on the upper right side of the IDEXX
VetLab Station title bar, the LED on the front panel of the Catalyst One analyzer ashes red, and the Catalyst
One icon on the IDEXX VetLab Station Home screen appears with an Alert status.
To View an Alert
Do one of the following:
+ Tap the Catalyst One icon on the IDEXX VetLab Station Home screen.
+ Tap the alert message in the title bar to display the alert message. Follow the instructions displayed in
the alert message.
Installing the Catalyst One Analyzer
The Catalyst One analyzer works in conjunction with the IDEXX VetLab Station.
To Install the Catalyst One Analyzer
1. Before you unpack the analyzer, choose an optimum location for the instrument. The analyzer should
be placed on a level surface in a well-ventilated area, away from obvious sources of heat, direct sunlight,
cold, humidity, or vibrations, and with 2 inches of ventilation around the analyzer. For optimum results,
room temperature should be at 15°C–30°C (59°F–86°F) and relative humidity at 15%–75%.
IMPORTANT: Ensure proper ventilation. The analyzer’s cooling vents are in the base and the back.
2. Use the Ethernet cable provided to connect the analyzer to a numbered port on the IDEXX VetLab router.
Note: For more information about connecting your analyzer to the router, see the installation instructions
that accompanied your router.
3. Power on the Catalyst One analyzer. Once the Catalyst One icon displays on the IDEXX VetLab Station
Home screen, your connections are complete.
Note: If the Catalyst One icon does not appear on the IDEXX VetLab Station Home screen within 3
minutes, contact IDEXX Technical Support for assistance.
Getting Started
‡
Feature coming soon

12
Catalyst One Analyzer Consumables
The following consumables are available for use with the Catalyst One analyzer:
CLIPs, Panels, and Slides
You can run any IDEXX slide on any species; however, reference intervals may not always be provided (see
footnotes for more information).
Chemistry Abbreviation
Chem 17 CLIP
Chem 15 CLIP
Chem 10 CLIP
Equine 15 CLIP
NSAID 6 CLIP
UPC Panel
†
Lyte 4 CLIP
QC CLIP
Individual Slides
Albumin ALB
Alkaline Phosphatase ALKP
Alanine Aminotransferase ALT
Amylase AMYL
Aspartate Aminotransferase AST
Bile Acids
†
BA
Blood Urea Nitrogen BUN
Calcium Ca
Cholesterol CHOL
Creatine Kinase CK
Creatinine CREA
Chloride Cl
C-Reactive Protein
‡
CRP
Fructosamine
†
FRU
Gamma-glutamyltransferase GGT
Glucose GLU
Potassium K
Lactate LAC
Lactate Dehydrogenase LDH
Lipase LIPA
Magnesium Mg
Sodium Na
Ammonia NH
3
Phenobarbital
†
PHBR
Inorganic Phosphate PHOS
Pancreatic Lipase
†
PL
Progesterone
‡
PROG
Symmetric dimethylarginine
†
SDMA
Total Bilirubin TBIL
Total Protein TP
Total T
4
†
TT4
Getting Started

13
Chemistry Abbreviation
Chem 17 CLIP
Chem 15 CLIP
Chem 10 CLIP
Equine 15 CLIP
NSAID 6 CLIP
UPC Panel
†
Lyte 4 CLIP
QC CLIP
Individual Slides
Triglycerides TRIG
Urine Creatinine UCRE
Urine Protein UPRO
Uric Acid URIC
†
Validated reference intervals for equine and “other” species are unavailable.
‡
Validated reference intervals for feline, equine, and “other” species are unavailable.
Compatible Species
Species with specic reference intervals:
Canine
†
Bovine
Feline
†
Llama
Equine
†
Sea Turtle
†
Species-specic intervals are available for these species. All other species are qualied as “other.”
Groups of species with guideline reference intervals:
Note: Guideline reference intervals will vary because there is diversity within the species of these groups.
Avian Monkey Rat
Ferret Mouse Sheep
Goat Pig Snake
Lizard Rabbit Tortoise
Getting Started
14
Using the Catalyst One* Analyzer
Analyzing Samples
All Catalyst One* analyzer runs are initiated via the IDEXX VetLab* Station. The process for doing so varies
depending on if your IDEXX VetLab Station is integrated with a practice information management system
(PIMS). For detailed instructions on initiating a sample run, see the IDEXX VetLab Station Operator’s Guide.
Slide Handling
The Catalyst One analyzer allows you to run up to 25 tests on a single sample. Before you begin, please take
note of the following:
+ Frozen CLIPs/panels/slides can be run on the Catalyst One analyzer (no thawing required).
+ Most CLIPs/slides should be loaded within 5 minutes of opening their foil packaging. The Catalyst* Lyte
4 CLIP and Catalyst* Pancreatic Lipase should be loaded within 2 minutes of opening its foil packaging.
+ If you are running a Lyte 4 CLIP, be sure to load it in the sample drawer before any other CLIPs or slides.
+ For optimal time to results, the recommended load order is Lyte 4 CLIP on the bottom, followed by a
chemistry CLIP (e.g., Chem 17, Chem 10, etc.), any additional slides, and TT
4
on top.
Diluting Samples
Dilutions should only be performed when a test value is outside the reportable range or when the sample
contains interfering substances (e.g., medications) that cause a nonlinear or invalid result. The Catalyst
One analyzer supports automated dilutions (the analyzer mixes the sample and diluent for you) and manual
dilutions (you prepare the dilution outside of the analyzer). To initiate a dilution, on the Select Instruments
screen tap the Catalyst One Analyzer icon and specify the dilution information.
Remember the following important notes when diluting samples for analysis on the Catalyst One analyzer:
+ Only dilute tests with results outside of the reportable range. Diluting tests with results in the normal
range may produce invalid results.
+ All chemistries should be analyzed rst on the undiluted sample. Some analytes, such as GGT and total
bilirubin, have low serum/plasma concentrations. These analytes may be diluted out even with the lowest
dilution. Dilute the remaining sample and analyze any chemistries that were outside of the reportable
range on the rst analysis.
+ Perform a dilution only when a test value is accompanied by a greater-than symbol (>) or when the
analyzer informs you a dilution is necessary to receive accurate results.
+ Use the proper diluent material for your sample type.
– For plasma and serum samples, use normal saline.
– IDEXX does not recommend manually diluting whole blood in a Catalyst* whole blood separator—
only dilute the separated plasma.
– For urine, use
Catalyst* Urine P:C Diluent.
+ Use an accurate measuring device, such as a calibrated pipette or syringe.
+ For best results, start with a 1:2 dilution (1 part sample to 1 part diluent)—do not exceed 9 parts diluent.
+ Do not perform a manual or automated dilution on electrolytes, NH
3
, PHBR, TT
4
, SDMA, PL, FRU, BA, or
PROG tests, or on whole blood samples.

15
+ Do not dilute small samples to achieve a minimum sample volume. Such dilutions on normal analyte
concentration cannot be read accurately. When dilution is needed to determine some analytes at very
high concentration, the sample should be diluted manually.
+ An automated dilution run will be canceled if:
– There is insucient diluent/sample volume.
– There are too many slides in the run.
Minimum Sample Volume for Dilutions
The minimum sample volume varies based on the dilution factor and the number of slides that are being
diluted (see table below).
Parts Sample +
Parts Diluent =
Diluent Ratio
Maximum
Number of
Slides per
Dilution
Minimum Sample Volume Diluent Volume
Serum, Plasma,
or Urine
Whole Blood
1 + 1 = 1:2 5 155 µL 700 µL 300 µL
1 + 3 = 1:4 10 130 µL 700 µL 300 µL
1 + 5 = 1:6 10 130 µL 700 µL 300 µL
1 + 9 = 1:10 10 100 µL 700 µL 300 µL
Preparing
Manual Dilutions
To Prepare a 1:2 Dilution
1. Accurately measure the desired amount of sample to be diluted and gently transfer it to a sample cup.
2. Accurately measure an equal amount of diluent and transfer it to the sample collected in step 1.
3. Thoroughly mix the sample and diluent.
4. Analyze the sample.
To Prepare Dilutions Greater Than 1:2
If additional dilutions beyond 1:2 are necessary, always begin with the original, undiluted sample. Then,
incrementally increase the parts diluent as indicated in the dilution chart (below).
Volumes are for example only. Parts Sample + Parts Diluent = Total Parts (Dilution Factor)
Parts Sample Parts Diluent Total Parts
(Dilution Factor)
1 (100 µL) 0 1 (undiluted sample)
1 (100 µL) 1 (100 µL) 2
1 (100 µL) 2 (200 µL) 3
1 (100 µL) 3 (300 µL) 4
1 (100 µL) 4 (400 µL) 5
1 (100 µL) 5 (500 µL) 6
1 (100 µL) 6 (600 µL) 7
1 (100 µL) 7 (700 µL) 8
1 (100 µL) 8 (800 µL) 9
1 (100 µL) 9 (900 µL) 10
Using the Catalyst One* Analyzer

16
Viewing and Printing Test Results
Analyzer results are automatically returned to the IDEXX VetLab Station and recorded in the appropriate
patient’s record. The diagnostic results report is a comprehensive report of all the test results specied in a
laboratory request for that patient on a specic day.
Patient test results can be printed automatically each time a set of results are returned or you can manually
print the results when needed.
For more information about how to view and print test results, see the IDEXX VetLab Station Operator’s
Guide.
Outside of Reportable Range Samples
Occasionally a test value may be outside the analyzer’s reportable range capability. The test value may be
greater than (“>”) the reportable range, or interfering substances in the sample may be causing a nonlinear
or invalid result. See the following chart for reportable ranges on individual chemistries. If a value is
required, it will be necessary to dilute the sample and repeat the test.
Chemistry U.S. Units S.I. Units French Units
ALB 0.1–6.0 g/dL 1–60 g/L 1–60 g/L
ALKP 10–2,000 U/L 10–2,000 U/L 10–2,000 U/L
ALT 10–1,000 U/L 10–1,000 U/L 10–1,000 U/L
AMYL 5–2,500 U/L 5–2,500 U/L 5–2,500 U/L
AST 0–1,083 U/L 0–1,083 U/L 0–1,083 U/L
BA 1.0–180.0 µmol/L 1.0–180.0 µmol/L 1.0–180.0 µmol/L
BUN/UREA 2–130 mg/dL 0.6–46.4 mmol/L 0.034–2.730 g/L
Ca 1.0–16.0 mg/dL 0.25–4.00 mmol/L 10–160 mg/L
CHOL 6–520 mg/dL 0.16–13.44 mmol/L 0.06–5.20 g/L
CK 10–2,036 U/L 10–2,036 U/L 10–2,036 U/L
Cl
‡
50–160 mmol/L 50–160 mmol/L 50–160 mmol/L
CREA 0.1–13.6 mg/dL 9–1202 µmol/L 1.0–136.0 mg/L
CRP 0.1–10.0 mg/dL 1.0–100.0 mg/L 1.0–100.0 mg/L
FRU
‡
100–1,000 µmol/L 100–1,000 µmol/L 100–1,000 µmol/L
GGT 0–952 U/L 0–952 U/L 0–952 U/L
GLU 10–686 mg/dL 0.56–38.11 mmol/L 0.10–6.86 g/L
K
‡
0.8–10 mmol/L 0.8–10 mmol/L 0.8–10.0 mmol/L
LAC 0.50–12.00 mmol/L 0.50–12.00 mmol/L 0.50–12.00 mmol/L
LDH 50–2,800 U/L 50–2,800 U/L 50–2,800 U/L
LIPA 10–6,000 U/L 10–6,000 U/L 10–6,000 U/L
Mg 0.5–5.2 mg/dL 0.21–2.17 mmol/L 5.0–52.0 mg/L
Na
‡
85–180 mmol/L 85–180 mmol/L 85–180 mmol/L
NH
3
‡
0–950 µmol/L 0–950 µmol/L 0–950 µmol/L
PHBR
†‡
5–55 µg/mL 5–55 µg/mL 5–55 µg/mL
PHOS 0.2–16.1 mg/dL 0.06–5.19 mmol/L 2.00–161.00 mg/L
PL (canine)
‡
30–2,000 U/L 30–2,000 U/L 30–2,000 U/L
PL (feline)
‡
0.5–50 U/L 0.5–50 U/L 0.5–50 U/L
PROG
‡
0.2–20.0 ng/mL 0.6–63.6 nmol/L 0.2–20.0 ng/mL
SDMA
‡
0–100 µg/dL 0–100 µg/dL 0–100 µg/dL
Using the Catalyst One* Analyzer

17
Chemistry U.S. Units S.I. Units French Units
TBIL 0.1–27.9 mg/dL 2–477 µmol/L 1.0–279.0 mg/L
TP 0.5–12.0 g/dL 5–120 g/L 5–120 g/L
TRIG 10–375 mg/dL 0.11–4.23 mmol/L 0.10–3.75 g/L
TT
4
(canine)
‡
0.5–10.0 µg/dL 6.43–128.70 nmol/L 6.4 3–128.70 nmol/L
TT
4
(feline)
‡
0.5–20.0 µg/dL 6.4–257.4 nmol/L 6.4–257.4 nmol/L
UCRE 6–350 mg/dL 0.06–3.50 g/L 0.06–3.50 g/L
UPRO 5–400 mg/dL 0.05–4.00 g/L 0.05–4.00 g/L
URIC 0.1–20 mg/dL 6–1,190 µmol/L 1–200 mg/L
†
1 µg/mL = 4.31 µmol/L
‡
Indicates sample types that should not be diluted.
Using the Catalyst One* Analyzer
18
Modifying the Settings on the
Analyzer
Modifying the Sound Settings
‡
The analyzer will beep when it encounters an alert. You can modify the Sound settings to turn the sound off
or adjust its volume.
1. Tap the Catalyst One icon on the IDEXX VetLab Station Home screen.
2. If you do not want the analyzer to make any sounds, tap Off in the Sound area.
OR
3. If you want the volume of the sound to be quiet, tap Low in the Sound area.
OR
4. If you want the volume of the sound to be loud, tap High in the Sound area.
Entering Standby Mode
You can modify the settings of the analyzer so that it enters Standby mode at a certain time each day or
put it in Standby mode immediately.
1. Tap the Catalyst One icon on the IDEXX VetLab Station Home screen.
2. If you do not want the analyzer to ever enter Standby mode, tap Never in the Standby area.
OR
3. If you want the analyzer to enter Standby mode at a certain time each day, tap Daily in the Standby area
and then select the desired start time from the available drop-down list.
OR
4. If you want the analyzer to enter Standby mode immediately, tap Now in the Standby area.
Exiting Standby Mode
You can set the analyzer to exit Standby mode at a certain time each day or immediately.
1. Tap the Catalyst One icon on the IDEXX VetLab Station Home screen.
2. If you want the analyzer to exit Standby mode at a certain time each day, tap Daily in the Exit Standby
area and then select the desired start time from the available drop-down list.
OR
3. If you want the analyzer to exit Standby mode immediately, tap Now in the Exit Standby area.
‡
Feature coming soon

19
Sample Preparation and Storage
Supported Sample Types for Catalyst* CLIPs and Slides
The following sample types can be used with Catalyst* CLIPs and slides:
CLIPs/Slides Abbreviation
Serum
Lithium Heparin-
Treated Plasma
Fluoride/
Oxalate-Treated
Plasma
Untreated Whole
Blood (using the
Catalyst* Lithium
Whole Blood
Separator)
Urine
Chem 17 CLIP N/A
Chem 15 CLIP N/A
Chem 10 CLIP N/A
Equine 15 CLIP N/A
NSAID 6 CLIP N/A
UPC Panel N/A
Lyte 4 CLIP N/A
Albumin ALB
Alkaline Phosphatase ALKP
Alanine Aminotransferase ALT
Amylase AMYL
Aspartate Aminotransferase AST
Bile Acids BA
Blood Urea Nitrogen BUN/UREA
Calcium Ca
Cholesterol CHOL
Creatine Kinase CK
Creatinine CREA
C-Reactive Protein CRP
Fructosamine FRU
Gamma-glutamyltransferase GGT
Glucose GLU
Lactate LAC
Lactate Dehydrogenase LDH
Lipase LIPA
Magnesium Mg
Ammonia NH
3
Phenobarbital PHBR
Inorganic Phosphate PHOS
Pancreatic Lipase PL
Progesterone PROG

20
CLIPs/Slides Abbreviation
Serum
Lithium Heparin-
Treated Plasma
Fluoride/
Oxalate-Treated
Plasma
Untreated Whole
Blood (using the
Catalyst* Lithium
Whole Blood
Separator)
Urine
Symmetric dimethylarginine SDMA
Total Bilirubin TBIL
Total Protein TP
Total T
4
TT
4
Triglycerides TRIG
Uric Acid URIC
Preparing Samples for Use on the Catalyst One Analyzer
You can run untreated whole blood, lithium heparinized whole blood, plasma, serum, and urine samples on
the Catalyst One analyzer.
IMPORTANT: Do not use EDTA or sodium heparin for chemistry analysis.
To Prepare an Untreated Whole Blood Sample
(Using a Lithium Heparin Whole Blood Separator)
1. Remove the green cap from the lithium heparin whole blood separator to prepare it for sample collection.
2. Immediately after sample collection (to avoid clotting), dispense 0.7 cc of untreated (no additive) whole
blood into the lithium heparin whole blood separator using an untreated syringe with the needle removed.
Tip: Use the ll line on the separator to ensure proper ll volume.
Note: Heparinized samples can be used in the lithium heparin whole blood separator except when running
feline AST, LDH, or CK. Double dosing may elevate the results for these assays in feline samples.
3. Gently swirl (do not invert or shake) the whole blood separator at least 5 times to mix the sample with
the anticoagulant.
Caution: Ensure that the cap is removed before loading the separator into the analyzer.
1 32
Fill to lowest line on
separator (0.7 cc [700 µL])
To Prepare a Plasma Sample
1. Use the appropriate tube and collection device.
2. Draw the sample gently and transfer if necessary.
Note: Be sure to use the correct blood-to-lithium heparin ratio.
3. Gently invert (do not shake) the sample for 30 seconds to mix.
4. As soon as possible (within 30 minutes of collection), centrifuge the sample at the appropriate setting
(refer to your centrifuge operator’s guide for settings and times).
Sample Preparation and Storage

21
5. Immediately after centrifugation, use a transfer pipette (or a 300 µL pipette) to transfer the appropriate
volume of sample to a Catalyst sample cup (ensure there are no bubbles in the sample cup and take
particular care not to aspirate cells during plasma collection). The volume needed varies depending on
the number of slides being used in the run—for more information, see “Proper Sample Cup Volume.”
2 3 4 5
To Prepare a Serum Sample
1. Use the appropriate tube and collection device.
2. Draw the sample gently and transfer if necessary.
3. Let the sample clot for a minimum of 20 minutes.
4. Within 45 minutes of collection, centrifuge the sample (refer to your centrifuge operator’s guide for
settings and times).
5. Immediately after centrifugation, use a transfer pipette (or a 300 µL pipette) to transfer the appropriate
volume of sample to a Catalyst sample cup (ensure there are no bubbles in the sample cup and take
particular care not to disturb the clot during serum collection). The volume needed varies depending on
the number of slides being used in the run—for more information, see “Proper Sample Cup Volume.”
2 3 4 5
To Prepare a Urine Sample
1. Obtain the sample through cystocentesis (recommended), catheter, or free-catch method.
2. Transfer the sample to a disposable sample tube.
3. Centrifuge the sample.
4. Use a transfer pipette (or a 300 µL pipette) to transfer the appropriate volume of supernatant urine
to a Catalyst sample cup (ensure there are no bubbles in the sample cup). The volume needed varies
depending on the number of slides being used in the run—for more information, see “Proper Sample Cup
Volume.”
2 3 4
Sample Preparation and Storage

22
Sample Preparation and Storage
Proper Sample Cup Volume
When using a Catalyst Sample Cup, 300 microliters of serum or plasma will allow you to run most test
combinations. The following table provides general guidance for tests that do not include a reagent
consumable. Refer to applicable quick reference guides for test-specic sample type and volume
requirements.
Number of slides Sample cup fill volume (µL)
1 60
2 70
3 80
4 90
5 100
6 110
7 120
8 130
9 190
10 200
11 210
12 220
13 230
14 240
15 250
16 260
17 270
18 280
Sample Inspection After Centrifugation
It is good practice to examine the sample carefully following centrifugation in a centrifuge
and/or in the analyzer (by running a whole blood separator). If brin strands can be seen in the sample, they
may interfere with sample pipetting. It may be necessary to rim the serum/plasma with a wooden stick,
respin the sample, and proceed.
Various conditions, such as hemolysis, may affect results. You might also want to modify your test panel
based on the following visual observations. Refer to the “Chemistry Descriptions” section for information
about how each condition may affect specic chemistries.
Note: When using the Catalyst whole blood separator, we recommend that you inspect the sample after the
run for the conditions listed below and interpret the results accordingly.
Hemolysis
Visual: Sample has a transparent reddish hue ranging from pale pink to deep red.
Indications: Damage to red blood cells during sample preparation or intravascular
hemolysis.
Icterus
Visual: Plasma has a transparent yellow to opaque brown color.
Indications: Obstructive or toxic liver disease, intravascular hemolysis.

23
Lipemia
Visual: Sample has a pale, milky appearance, possibly with oating fat globules.
Indications: Recent ingestion of a fatty meal or dysfunction in lipid metabolism.
Sample Storage
We recommend that you prepare and analyze samples immediately after collection for best results.
However, if storage is necessary, follow these sample storage and testing guidelines.
Storing Serum/Plasma
For storage, the serum or plasma must be separated and removed immediately from the blood cells. Do
not attempt to pour off the sample.
+ Using a transfer pipette, carefully transfer the serum or plasma to an untreated collection tube, taking
care not to draw up any white or red blood cells.
+ Cap the tube tightly to avoid contamination and evaporation. Avoid frothing at any stage as this
damages the serum proteins.
If you cannot perform analysis within 4 hours of drawing and processing the sample, refrigerate the sample
immediately after preparation at 2°C–8°C (36°F–46°F). If you cannot analyze the refrigerated sample within
48 hours, you should freeze the serum/plasma at -18°C (0°F). Serum/plasma can be frozen immediately
after preparation and stored for up to 1 month.
Notes:
+ For additional information on the effects of delays in removing serum or plasma from the cells, see the
“Chemistry Descriptions” section.
+ See the calcium (Ca), total bilirubin (TBIL), lactate dehydrogenase (LDH), ammonia (NH
3
), electrolytes
(Na, K, Cl), and glucose (GLU) chemistry descriptions for additional special handling and storage
requirements.
+ IDEXX does not recommend freezing samples that will be used to run electrolytes, PROG, TT
4
, SDMA,
BA, or NH
3
.
Storing Whole Blood
Lithium heparinized whole blood samples should be analyzed immediately. Samples that will not be
analyzed within 30 minutes should be placed in a tube to be separated and stored (see instructions above).
Important: Do not store whole blood samples in whole blood separators.
Storing Urine
Urine should be tested within 2 hours. Do not store urine in the refrigerator for more than
24 hours. Urine should not be stored in the freezer.
Analysis of Stored Samples
For samples stored at 2°C–8°C (36°F–46°F) and at -18°C (0°F):
+ Allow the samples to come to room temperature (19°C–27°C/66°F–81°F).
+ Mix the samples gently, but thoroughly, by inversion. Do not shake.
+ Centrifuge the samples to remove any brin particles (or urine sediment) that may have formed during
storage.
+ Analyze the samples immediately after centrifugation.
Sample Preparation and Storage
24
Quality Control
Overview
The purpose of quality control (QC) is to verify the integrity of your slides and also to verify that your
Catalyst One* analyzer is functioning properly.
You should run a QC test:
+ When the analyzer is rst installed.
+ After cleaning the internal components of the analyzer.
+ If the analyzer has been moved.
+ To verify system performance.
Quality Control Materials
VetTrol* Control
In each box of VetTrol* Control, there are four vials containing freeze-dried powder (dark brown bottle
marked “VetTrol Control”) and four vials containing diluent (lighter bottles marked “Diluent for VetTrol”). The
lot numbers for the diluent and the control are different and can be found on the product packaging.
For more information about VetTrol Control, see its package insert.
Storage
Control and diluent vials should be stored frozen (-18°C/0°F). Discard opened control vials within 24 hours.
Expired or unwanted material should be discarded with other clinical waste.
Note: Do not store in the freezer door; only in the main freezer compartment.
Stability and Handling
For most chemistries, VetTrol Control can be used up to 24 hours after reconstitution when it is stored in
the refrigerator and equilibrated to room temperature before running (do not leave at room temperature for
more than 2 hours). For creatine kinase and ammonia values, VetTrol Control uid should be used within
2 hours following reconstitution. Exposure to light will affect total bilirubin and creatine kinase results.
Ammonia concentration will increase with time.
UPRO Control
In each box of UPRO Control, there are six vials containing the control uid. The lot number can be found on
the product packaging.
Storage
Control uid should be refrigerated (2°C–8°C/36°F–46°F). Discard at the expiration date. Expired or
unwanted material should be discarded with other clinical waste.
Stability and Handling
Use within 24 hours after opening (refrigerate when not in use).
25
Advanced Control
In each box of Advanced Control, there is one vial containing the control uid. The lot number can be found
on the product packaging.
Note: Each vial contains enough uid for 2 runs, in the event a secondary run is necessary.
Storage
Store frozen until the expiration date, or store in the refrigerator for up to 5 days.
Stability and Handling
Once opened, Advanced Control cannot be stored and reused—discard remaining uid after use.
PHBR Control
In each box of PHBR Control, there are six vials containing the control uid. The lot number can be found on
the product packaging.
Storage
Store frozen until the expiration date, or store in the refrigerator for up to 7 days.
Stability and Handling
Once thawed, PHBR Control cannot be stored and reused—discard remaining uid after use.
Quality Control CLIPs and Slides
IDEXX recommends that you perform monthly quality control testing after you have cleaned the internal
components of your analyzer. The convenient Catalyst* QC CLIP contains all of the chemistry slides
needed to perform this task. It is also recommended that you perform a quality control for electrolytes using
the Catalyst* Lyte 4 CLIP.
Run the QC CLIP and the Lyte 4 CLIP
Use the convenient QC CLIP and the Lyte 4 CLIP in conjunction with the VetTrol Control uid to perform
quality control on your Catalyst One analyzer. It is recommended that you wait at least 30 minutes after
running any slides before running the QC CLIP.
OR
Run Individual Slides
You can use individual slides to create your own QC panel and perform a quality control test (one slide per
group). If you want to use individual slides to run quality control, we recommend a minimum of one slide
from each of the groups below.
Group 1 NH
3
Group 2 AMYL
CHOL
GLU
LAC
LIPA
TBIL
TP
TRIG
Quality Control
26
Group 3 ALB
CREA
Mg
PHOS
BUN/UREA
URIC
UCRE
Group 4 ALT
LDH
Group 5 ALKP
GGT
Group 6 AST
Ca
CK
UPRO (to be used with UPRO Control uid only)
Preparing Control Fluid
The instructions for preparing control uid vary depending on the type of control you are preparing.
To Prepare VetTrol Control Fluid
1. Remove one diluent and one control vial from freezer. Allow 60–90 minutes for vials to acclimate to
room temperature.
2. Slowly invert the diluent vial several times, or place the vial on a tube rocker, to thoroughly mix the
contents. Do not shake.
3. Gently tap the control vial on the counter several times to dislodge any material adhering to the stopper.
4. Remove the seal and stopper from each vial just before adding the diluent to control. Do not leave the
vials open.
5. Transfer exactly 3.0 mL of diluent to the control vial, using a clean, dry, Class A volumetric pipette or an
equivalent automatic pipette. Discard the remaining diluent.
IMPORTANT: Measurement must be precise or results will be incorrect.
Note: If using a syringe, be sure to remove the needle.
6. Replace the stopper on the control vial and hold it rmly in place. Gently invert the vial 6–10 times every
10 minutes for 1 hour (the use of a slow rocker is recommended). Do not shake. Reconstitution, with
occasional inversion, will take 45–60 minutes. Visually verify that all freeze-dried material is dissolved
before using.
7. Run quality control on the Catalyst One analyzer (see instructions below).
To Prepare UPRO Control Fluid
1. Take one vial of UPRO Control out of the refrigerator and gently invert it 6–10 times to mix thoroughly.
2. Transfer 300 μL of UPRO Control into a Catalyst* sample cup (to be loaded in the sample drawer).
3. Let the contents in the sample cups reach room temperature (approximately 10 minutes).
4. Run quality control on the analyzer.
Quality Control
27
To Prepare Advanced Control Fluid
1. If the Advanced Control has been frozen, allow it to thaw for 30 minutes prior to use.
2. Invert the Advanced Control vial at least 5 times.
3. Transfer the contents of the Advanced Control vial to a Catalyst* sample cup.
4. Run quality control on the analyzer.
To Prepare PHBR Control Fluid
1. Take one vial of PHBR Control out of the freezer and allow it to reach room temperature (approximately
60 minutes).
2. Once you have conrmed that there is no visible frozen material in the vial, gently invert it 6–10 times to
mix thoroughly.
3. Transfer 300 μL of PHBR Control into a Catalyst* sample cup.
Note: You will need one PHBR slide wash and one PHBR slide for the quality control procedure.
4. Run quality control on the analyzer.
Running Quality Control
To Run General Quality Control on the Catalyst One analyzer
1. Tap the Catalyst One icon on the IDEXX VetLab Station Home screen.
2. Tap Maintenance and then tap Quality Control.
3. Tap the quality control lot number you are using and then tap Run QC.
4. Follow the on-screen instructions for preparing and running quality control.
Notes:
+ To view QC results at any time, tap Maintenance, tap Quality Control, tap View QC Results, select the
desired date that QC was run, and then tap View Results.
+ To view the expected ranges for each chemistry in a QC lot, tap Maintenance, tap Quality Control, select
the desired QC lot, and then tap View QC Lot Information.
Quality Control

28
Maintenance
Overview
In addition to performing monthly quality control checks on the Catalyst One* analyzer, it is recommended
that you:
+ Clean the analyzer internally and externally.
+ Upgrade the software promptly.
Upgrading the Software
As new features and functionality are added to the Catalyst One analyzer, you will receive software
upgrades from IDEXX. If you have IDEXX SmartService* Solutions, the upgrade will be sent via your IDEXX
VetLab* Station automatically. If you do not have SmartService Solutions, you will receive your upgrade in
the mail. Be sure to read the software notes contained with each new release.
Cleaning the Internal Components of the Analyzer
To ensure optimal performance of your analyzer, it is important that you clean the internal components
(incubator ring, optics window, and carousel) monthly and before performing quality control.
It is recommended that you wear clean powder-free latex or nitrile gloves when cleaning the internal
components of the analyzer. Wearing these gloves helps to avoid smudges on the components and
ensures an effective cleaning.
IMPORTANT: Never use cleaning materials (such as alcohol cleaning wipes containing sodium
bicarbonate) that leave a residue once the alcohol/solvent evaporates.
To Clean the Internal Components
1. Tap the Catalyst One icon on the IDEXX VetLab Station Home screen.
2. Tap Maintenance, tap Clean, and follow these on-screen instructions.
a. Open the side door on your analyzer.
b. Raise the carousel cover until the green lever magnetizes itself to the
inside of the analyzer.
c. Lift up on the carousel and remove it from the analyzer.
d. Using an IDEXX-supported alcohol prep pad, wipe the incubator ring and
optics window in a counterclockwise direction. Repeat this step at least
three times using a new alcohol prep pad for each wipe.
e. Clean the white reference tile using a new alcohol prep pad.
f. Using a dry optical tissue, dry the optics window and reference tile,
ensuring all signs of dampness have evaporated from the cleaned
components. If streaks or smudges remain, repeat the cleaning process.
g. Replace the carousel inside of the analyzer, lower the carousel cover and
close the side door.
h. Tap Done.
2c
2b
2e
29
Cleaning the Outside of the Analyzer
and the Sample Drawer
Clean the outside of the analyzer or sample drawer with a damp (not wet) lint-free cloth. A mild liquid soap
will remove grease. Do not use any of the following near the analyzer: organic solvents, ammonia-based
cleaning products, ink markers, sprays containing volatile liquids, insecticides, disinfectant, polish, or room
freshener.
Care should be taken not to spill any samples, chemicals, cleaning agents, water, or other uids
on/in the analyzer.
Note: Dust and animal hair can lead to analyzer failures. Routinely dust off the analyzer with a damp cloth
and dust around its location. Do not block the cooling vents under the analyzer by allowing paper, loose
materials, or dust to accumulate.
WARNING: Never wipe the analyzer or its surroundings with ammonia-based cleaning products. Avoid
urine odors around analyzer. Ammonia in the atmosphere will falsely increase ammonia (NH
3
)quality
control and patient test results.
Emptying the Waste Drawer
It is essential that you empty the waste drawer after every run or when prompted. The analyzer will not
operate when the waste drawer is full. Pull the waste drawer to remove it from the analyzer.
Maintenance
30
Appendices
Chemistry Descriptions
Serving veterinarians throughout the world, IDEXX Laboratories understands that medical content, including
interpretation of diagnostic results and medical protocols, may vary from country to country. A medical
review board has approved the content presented in this document.
IDEXX has more than 40 reference laboratories worldwide employing over 100 veterinarians. If you have
any questions about the medical content or interpretation of results in this document, please contact IDEXX
Laboratories.
Introduction to Biochemical Proling
By performing appropriate biochemical tests on quality samples, you can obtain information that, when
combined with patient history and clinical ndings, should assist you in making an accurate diagnosis.
Appropriate biochemical tests are also essential for monitoring and prognostication purposes once a
diagnosis is achieved.
Single tests are helpful in particular circumstances, such as following the course of an identied disease
or for monitoring the effect of therapy. However, many individual chemistry tests give information about
different organ systems and should be used in combination with other tests (panels or proles) to help
characterize disease.
Alanine Aminotransferase (ALT)
For practical purposes, the enzyme alanine aminotransferase is specic to the liver in dogs and cats.
It is found in the hepatocyte cytoplasm and may be released into the blood during both reversible and
irreversible (cell necrosis) changes.
Principal Reason for Performing the Test
To investigate hepatocellular injury in dogs and cats.
Note: This test is not useful in the detection of liver disease in ruminants, horses, and pigs as the enzyme
activity in the liver is very low. Even with severe liver disease in these species, the increase in activity is
minimal.
Most Common Abnormality Indicated by the Test
Hepatocellular injury.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. Hemolyzed specimens should not be used
because ALT contamination from red blood cells will occur. If plasma is being collected, use only lithium
heparinized samples.
Complementary Tests
Alanine aminotransferase activity is usually determined in conjunction with other tests of hepatic function
or damage.

31
Reaction Sequence
Albumin (ALB)
Albumin forms the largest fraction of the total serum protein in the healthy animal. It is synthesized
solely by the liver, has a relatively low molecular weight, and plays an important role in the transport of
endogenous and exogenous compounds by binding with those compounds. Albumin also plays a major
role related to osmoregulation.
Principal Reasons for Performing the Test
To investigate causes of hypoalbuminemia: protein-losing nephropathy, protein-losing enteropathy, as
well as hepatic insuciency (decreased production) and decreased absorption due to malabsorption
(gastrointestinal disease) or malnutrition. In addition, it is helpful in characterizing the degree of dehydration
with increases in serum albumin concentrations, and it is commonly decreased with active inammatory
disease (negative acute phase reactant).
The test should not be performed in isolation because of its lack of specicity.
Most Common Abnormalities Indicated by the Test
Decreased albumin—inammatory disease, protein-losing nephropathy and enteropathy, and decreased
production (hepatic insuciency).
Increased albumin—dehydration.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. Hemolysis may occur if the sample is not
handled properly. Although dry-slide technology minimizes the interfering effect of mild-to-moderate
hemolysis, marked hemolysis will cause an increased albumin value.
Complementary Tests
Albumin concentration is usually determined in conjunction with the measurement of total protein and
other tests of renal and hepatic function. When albumin is measured with total protein, the total globulins
will be calculated automatically and given with the results.
Reaction Sequence
Alkaline Phosphatase (ALKP)
The enzyme alkaline phosphatase is found in many body tissues. Highest levels are found in the kidney
cortex, small intestinal mucosa, and osteoblasts. The enzyme is also present in the liver primarily located
on the bile canalicular; thus an increase in ALKP may indicate cholestasis.
In cats and horses, the half-life of hepatic alkaline phosphatase is very short for ALKP and even shorter
for other natural tissue sources of ALKP due to rapid renal excretion/metabolism. Sensitivity of the test
in cats and horses is low. Since the nonhepatic sources of ALKP have relatively short half-lives compared
to the hepatic source, a mild-to-modest increase in ALKP in these species can be a specic indicator of
cholestasis.
Appendices

32
Principal Reason for Performing the Test
As an indicator of hepatic and/or biliary disease.
Most Common Abnormality Indicated by the Test
Obstructive changes in the biliary system. A special consideration for interpreting ALKP changes in the dog
is required because there are “induced” forms of ALKP due to glucocorticoids and other inuences that are
not associated with the natural tissue sources of ALKP. The nonhepatic sources of ALKP (bone, intestinal,
placental) in the dog will only rarely be measured as high as threefold above the high end of the reference
range because of their relative short half-lives compared to the induced and hepatic forms of ALKP. With
both the induced and hepatic source (cholestasis) of ALKP, serum enzyme activities are commonly greater
than the threefold increase; therefore, when a greater than threefold increase is noted in ALKP in the dog,
either cholestasis or induced enzyme is suspected.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is being collected, use only lithium
heparinized samples. Hemolyzed specimens should not be used because ALKP contamination from red
blood cells will increase results while hemoglobin decreases results. Above normal total bilirubin levels may
reduce ALKP results.
Complementary Tests
Alkaline phosphatase activity is usually determined in conjunction with other tests of hepatic function and
damage.
Reaction Sequence
Ammonia (NH
3
)
Ammonia is the catabolic product of protein digestion and is extremely toxic. It is converted rapidly in the
liver to urea, which is eliminated from the body by the kidneys.
Principal Reason for Performing the Test
To evaluate hepatic function.
Most Common Abnormality Indicated by the Test
Increased ammonia—decreased hepatic functional mass or hepatic vascular shunt.
Sample Type and Precautions
Use only lithium heparinized samples.
Blood should be processed and centrifuged immediately following collection; for this reason, plasma is
recommended as the sample of choice.
Ammonia measurements in either plasma or serum are signicantly affected by environmental factors
and/or the passage of time. Minimal exposure of the sample to the air is essential. All sample containers
should be capped unless sample is being introduced or withdrawn. Do not attempt to measure ammonia in
hemolyzed samples. Contamination from the red blood cells will invalidate the test.
Complementary Tests
Ammonia may be determined in isolation but more often in conjunction with other tests of hepatic damage
or dysfunction, such as pre- and postprandial bile acids.
Appendices

33
Reaction Sequence
Amylase (AMYL)
This section should be read in conjunction with the Lipase (LIPA) section.
The main source of serum amylase is the pancreas, although pathology of the liver and small intestine may
result in signicant elevations of this enzyme (above the reference range). Since amylase is cleared by the
kidneys, renal pathology may also result in elevation of amylase independent of pancreatic disease.
Principal Reason for Performing the Test
As an indicator of pancreatic disease and potential acute pancreatitis.
Most Common Abnormality Indicated by the Test
Acute necrotizing pancreatitis.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. Hemolyzed specimens should not be used. Do
not use oxalate, citrate, or EDTA anticoagulants. If plasma is being collected, use only lithium heparinized
samples.
Blood samples should be taken within one day of the onset of symptoms that suggest acute pancreatitis.
Complementary Tests
Amylase and lipase are usually determined in conjunction with one another. Evaluation of a comprehensive
chemistry prole that includes electrolytes is generally recommended because of secondary effects of
acute pancreatitis. Specic pancreatic lipase should be considered in suspected cases of pancreatitis.
Reaction Sequence
Aspartate Aminotransferase (AST)
The enzyme aspartate aminotransferase is present in large amounts in multiple tissues of dogs, cats, and
many other animal species. Hepatocytes, cardiac muscle cells, and skeletal muscle cells have relatively
high concentrations of AST. It is found in the cytoplasm and mitochondria of the cells and is released into
the blood during cell injury. If no increase in ALT is seen in conjunction with an increased AST in the dog
and cat, cardiac or skeletal muscle cell injury is most likely. For increased AST values with equine, bovine,
and porcine samples, liver, cardiac, and skeletal muscle cell injury must be considered.
Principal Reason for Performing the Test
To investigate damage to liver, cardiac, or skeletal muscle.
Most Common Abnormalities Indicated by the Test
Dogs and cats—cardiac or skeletal muscle injury when ALT is not increased; liver, cardiac, or skeletal
muscle injury if both ALT and AST are increased.
Horses, cows, and pigs —liver, cardiac, or skeletal muscle injury.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. Hemolyzed specimens should not be used
because AST contamination from red blood cells will occur. EDTA and uoride/oxalate should not be used as
anticoagulants. If plasma is being collected, use only lithium heparinized samples.
Appendices

34
Blood samples should be processed and centrifuged immediately after collection. Even slight hemolysis
can cause marked increases in activity because of high intracellular concentrations of AST in red blood
cells.
Complementary Tests
Aspartate aminotransferase activity is usually determined in conjunction with other tests of liver, cardiac, or
skeletal muscle function or damage.
Reaction Sequence
Bile Acids (BA)
Bile acids are produced in the liver, stored in the gallbladder, and released into the intestinal tract where
they aid in lipid digestion. In healthy animals, bile acids are eciently reabsorbed from the intestinal tract
and recirculated to the liver via the portal vein. Once in the liver, bile acids are removed from circulation by
the hepatocytes. In states of disease or abnormal portal blood ow, bile acids can become elevated in the
systemic circulation, indicating reduced liver function.
Principal Reason for Performing the Test
Bile acids testing is primarily used to evaluate for loss of liver function or presence of portosystemic
shunts; however, bile acids results can also be elevated with cholestatic diseases that cause bile retention.
Bile acids testing is particularly useful when there is suspicion of liver disease before more expensive or
invasive testing is performed (e.g., ultrasound, biopsy). Bile acids testing may also be useful for monitoring
the effects of some therapeutic drugs on hepatic function and as part of the evaluation for hepatic
encephalopathy in patients with neurologic signs. Please refer to the IDEXX Bile Acids Algorithm for
additional information.
Most Common Abnormalities Indicated by the Test
Elevated pre- and/or postprandial bile acids are suggestive of liver dysfunction. Normal bile acids do not
rule out the presence of hepatic disease. Mild elevations may also be seen with extrahepatic diseases (e.g.,
small intestinal bacterial overgrowth [SIBO], hyperadrenocorticism, etc.). Moderate to severe elevations are
consistent with hepatic dysfunction but cannot discriminate specic liver diseases or the relative severity or
reversibility of liver disease. For additional information see the Bile Acids differentials in VetConnect* PLUS.
Sample Types and Precautions
Catalyst Bile Acids supports the use of serum, lithium heparin plasma, and whole blood (using the Catalyst
Lithium Whole Blood Separator). Remove plasma or serum promptly from the cells or clot. IDEXX does not
recommend freezing samples that will be used to run Catalyst Bile Acids.
Appendices

35
+ Catalyst Bile Acids is robust to lipemia.
+ Moderate to marked hemolysis can result in elevated Catalyst Bile Acids results.
+ If the serum/plasma bilirubin concentration is elevated or the animal is icteric, there is little additional
diagnostic value in performing a bile acids test. Icteric samples may result in moderately elevated
Catalyst Bile Acids results.
+ Be careful not to aspirate cells during serum/plasma preparation, and ensure the Catalyst Lithium Whole
Blood Separator is lled with 0.7 cc to prevent overlling.
Stimulation testing that includes both pre- and postprandial samples collected using typical bile acids
stimulation protocols is recommended to increase sensitivity. The following bile acids stimulation protocol
is recommended:
1. Fast the dog or cat for approximately 12 hours and collect a fasting (preprandial) sample. Obtain a result
from the preprandial Catalyst Bile Acids test.
2. Feed the animal a small amount of high-fat food to stimulate gallbladder contraction.
– The minimum amount of food recommended is 2 tsp for small patients (<10 lb) and
2 tbsp for large patients.
– If encephalopathic effects of protein are anticipated, use a restricted-protein food mixed with a small
amount of corn oil.
3. Two hours after feeding, collect a postprandial sample. Obtain a result from the postprandial Catalyst
Bile Acids test.
Complementary Tests
Bile acids testing is most frequently utilized after abnormal results on a minimum database indicate
concern for liver dysfunction. When paired with appropriate clinical signs, abnormal results that may
prompt the need for bile acids testing include:
+ CBC (decreased MCV)
+ Chemistry (decreased albumin, BUN, glucose, or cholesterol; increased ALT, AST, ALKP, GGT, or
ammonia)
+ Urinalysis (ammonium biurate crystalluria)
If bilirubin concentration is elevated or the animal is icteric, there is little additional value in performing a bile
acids test.
Reaction Sequence
Bile acids
Oxidized bile acids
3--hydroxysteroid dehydrogenase
NADH + MT
T
NAD + Formazan
Diaphorase
NAD
+
NADH
Blood Urea Nitrogen (BUN)
The catabolism of proteins results in the production of ammonia, which is extremely toxic. Ammonia is
converted to urea in the liver and eliminated from the body by glomerular ltration in the kidneys.
Principal Reason for Performing the Test
As an indicator of renal disease or pathologic conditions that result in bleeding into the gastrointestinal
tract.
Appendices

36
Most Common Abnormalities Indicated by the Test
Increased urea—prerenal, postrenal, and renal azotemia with decreased glomerular ltration rate; high-
protein diet or bleeding into the gastrointestinal tract.
Decreased urea —decreased protein intake; hepatic insuciency; diuresis.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is being collected, use only lithium
heparinized samples.
Blood should not be drawn for urea determination within 6 hours of a meal. Do not use sodium uoride or
EDTA as anticoagulant. Samples that contain hemoglobin increase urea nitrogen.
Complementary Tests
Urea concentration should usually be determined in conjunction with measurements of creatinine,
inorganic phosphate, total protein, albumin, and a complete urinalysis. Urea concentration is inuenced by
high-protein diet rather than creatinine.
Reaction Sequence
Calcium (Ca)
Calcium is an essential element that is involved in many body systems. These include the skeleton, enzyme
activation, muscle metabolism, blood coagulation, and osmoregulation. In the blood, calcium exists in
ionized and protein bound forms. Factors governing the total plasma, whole blood, or serum concentration
are complex and include interaction with other chemical moieties, proteins, and hormones.
Calcium, phosphorus, and albumin metabolism are interdependent.
Principal Reason for Performing the Test
As an indicator of certain neoplasias, bone disease, parathyroid disease, eclampsia, and renal disease.
Most Common Abnormalities Indicated by the Test
Increased calcium—hypercalcemia of malignancy (due to tumor release of PTH-like substances), spurious.
Decreased calcium—potential renal failure with resultant hyperphosphatemia, dietary, spurious.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is being collected, use only lithium
heparinized samples.
Centrifugation should take place quickly after the sample has been drawn. The sample should not be
exposed to the air for long periods. Glassware must be scrupulously cleaned to avoid contamination by
sources of calcium (e.g., detergents). Prolonged contact with the clot may lead to lowered calcium values
due to dilution by red blood cell water.
Do not use tubes containing uoride, oxalate, citrate, or EDTA as these agents will cause signicant negative
interference due to calcium chelation.
If analysis cannot be performed within 4 hours, the sample should be removed from the red blood cells and
refrigerated in a tightly stoppered container at 2°C–8°C (36°F–46°F) for short-term storage (up to 24 hours).
The sample should not be frozen. The sample must be allowed to reach room temperature before analysis.
Appendices

37
Complementary Tests
Calcium should be determined in conjunction with measurements of inorganic phosphate, albumin, total
protein, and glucose. Ionized calcium measurement will provide more specic information related to the
physiologic form of calcium.
Reaction Sequence
Chloride (Cl)
Chloride is the major anion, predominantly in the extracellular spaces, where it maintains cellular integrity
by inuencing osmotic pressure. Chloride determination is signicant in monitoring
acid-base balance and water balance.
Principal Reason for Performing the Test
Low chloride levels are usually found in severe vomiting or diarrhea, ulcerative colitis, severe burns, heat
exhaustion, fever, and acute infections. Increased values are found in dehydration, hyperventilation, anemia,
and cardiac decompensation.
Most Common Abnormalities Indicated by the Test
Hyperchloremia—if increased with sodium then the same cause of hypernatremia. Without a concurrent
increase in sodium: hyperchloremic acidosis: GI or renal loss of HCO
3
.
Hypochloremia (without related change in sodium)—upper GI tract loss (vomiting).
Sample Type and Precautions
Avoid hemolysis—sample should be run as soon as possible after serum or plasma is separated from the
cells or clot. If plasma is being collected, use only lithium heparinized samples. Potassium bromide may
increase Catalyst electrolyte results.
Do not freeze samples for use with the Catalyst One analyzer.
Complementary Tests
Sodium, potassium, and chloride should always be assayed together to determine electrolyte balance. If
sodium, potassium, chloride, and bicarbonate are measured together, accurate assessment of metabolic
acid-base physiology is possible.
Reaction Sequence
Cholesterol (CHOL)
Serum cholesterol occurs predominantly at high concentration in the esteried form; the remainder is in
the free form. Cholesterol is synthesized in the liver and other tissues and is also absorbed in the free form
from the small intestine. It is esteried in the liver and is the precursor of steroid hormones.
Cholesterol is broken down in the liver to bile acids and eliminated via the bile duct.
Principal Reason for Performing the Test
May be a marker for cholestasis or endocrine disease, such as hypothyroidism, hyperadrenocorticism,
diabetes mellitis, as well as nephrotic syndrome.
Appendices

38
Most Common Abnormality Indicated by the Test
Increased cholesterol—hypothyroidism, postprandial, nephrotic syndrome.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. Blood should not be drawn within
12 hours of a meal. If plasma is being collected, use only lithium heparinized samples.
Complementary Tests
Cholesterol measurements should not be performed in isolation but as part of a prole of tests to
investigate endocrine, hepatic, and renal disease. If high cholesterol is found in the absence of diabetes,
hepatic, or renal disease, hypothyroidism may be present. This can be evaluated by measuring thyroid
function.
Reaction Sequence
Creatine Kinase (CK)
Creatine kinase is found at high activity only in the cytoplasm of cardiac and skeletal muscle. This enzyme
catalyzes the reversible phosphorylation of creatine by ATP to creatine phosphate and ADP. Creatine
phosphate is the major source of high-energy phosphate used in muscle contraction.
Principal Reason for Performing the Test
To identify injury to skeletal or cardiac muscle.
Most Common Abnormality Indicated by the Test
Skeletal muscle lesions attributable to trauma or vigorous exercise.
Sample Type and Precautions
Samples must be processed and centrifuged immediately after drawing blood. Blood samples should
be taken within 6 hours of a suspect lesion. It is important to determine that the patient has not been
exercised vigorously during the 12 hours prior to sampling. This may cause marked increases in creatine
kinase activity. Remove plasma or serum from the cells or clot. If plasma is being collected, use only lithium
heparinized samples. EDTA and uoride/oxalate will reduce creatine kinase results.
Complementary Tests
Creatine kinase determination provides a specic, sensitive indication of muscle cell damage. Aspartate
aminotransferase and lactate dehydrogenase activities may also be measured but are less specic and
show smaller corresponding increases when muscle damage is present.
Appendices
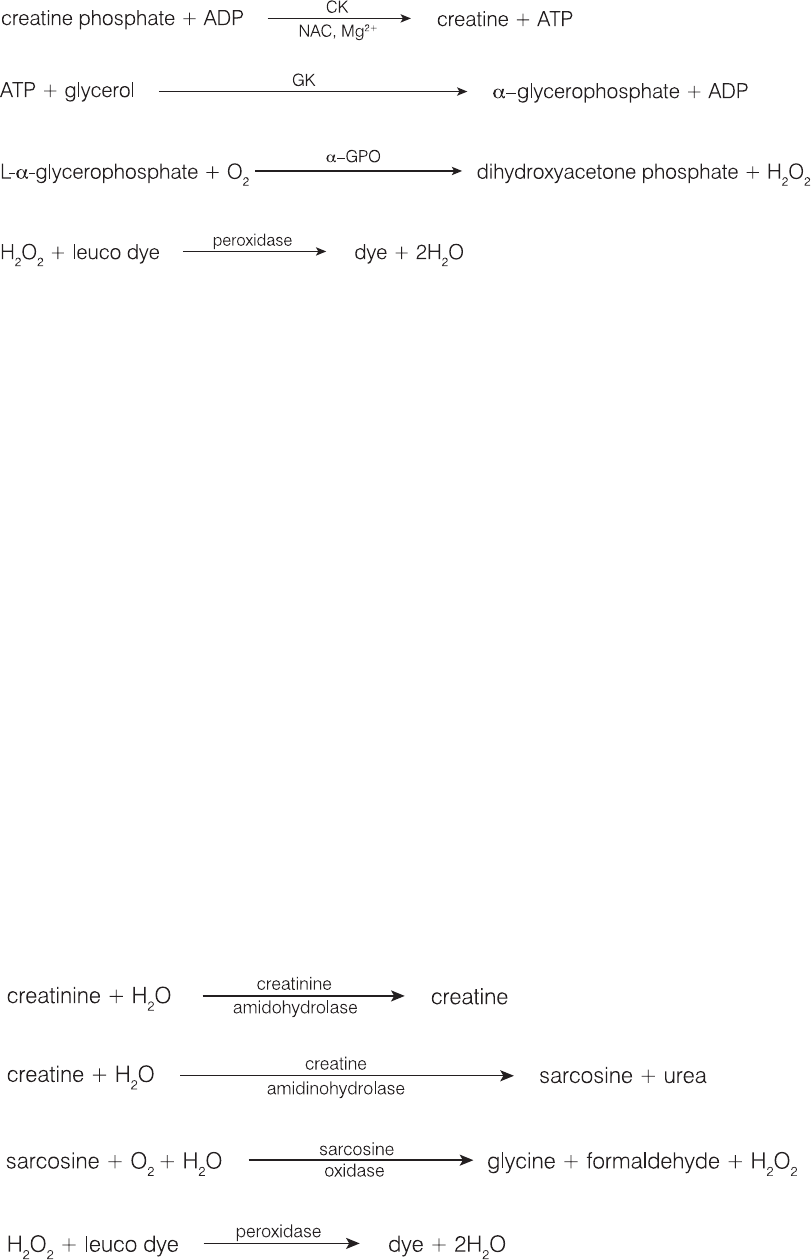
39
Reaction Sequence
Creatinine (CREA)
Creatinine is a degradation product of creatine in muscle metabolism. The daily production of creatinine
is fairly constant and not inuenced markedly by age, diet, exercise, or catabolism. Creatinine is eliminated
from the body by glomerular ltration and tubular secretion in the kidneys.
Principal Reasons for Performing the Test
As an indicator of renal disease and/or an index of glomerular ltration rate.
Most Common Abnormality Indicated by the Test
Increased creatinine—prerenal, postrenal, and renal azotemia.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is being collected, use only lithium
heparinized samples.
Interfering substances, such as creatine, in a sample can affect the analyzer’s ability to accurately provide
creatinine results. When the analyzer detects such an interfering substance, dilution of the sample may be
required to obtain an accurate creatinine value.
Complementary Tests
A complete urinalysis with a refractometry specic gravity measurement is essential for proper
interpretation of increases in creatinine. Creatinine determinations should usually be performed in
conjunction with measurements of BUN, inorganic phosphate, total protein, and albumin. A complete blood
count (CBC) can sometimes demonstrate changes such as nonregenerative anemia with chronic renal
failure.
Reaction Sequence
Appendices
40
C-Reactive Protein (CRP)
C-reactive protein (CRP) is the major acute phase protein released by the liver in response to systemic
inammation in selected species including the dog. The Catalyst CRP Test is a sandwich immunoassay
using monoclonal antibodies conjugated to gold nanoparticles and latex particles for the measurement of
CRP.
Principal Reason for Performing the Test
CRP is a highly sensitive biomarker of active systemic inammation in the canine patient. CRP will help
the veterinarian detect active inammation early, characterize the severity of the inammatory response,
and closely monitor the resolution or progression of the inammatory process following therapeutic
intervention.
Most Common Abnormality Indicated by the Test
CRP will be signicantly increased in any condition where active, systemic inammation is present. The
increase in CRP correlates with the severity of the inammation. An increased CRP value may be seen with
infectious and noninfectious inammatory disease (i.e., pneumonia, pancreatitis, pyelonephritis, pyometra,
septicemia, and pyothorax), immune-mediated disease (i.e., immune-mediated hemolytic anemia and
polyarthritis), as well as inammation associated with tissue injury as seen in major surgery.
Sample Type and Precautions
Samples acceptable for CRP measurement include serum, plasma, and whole blood (when using the
Catalyst Lithium Heparin Whole Blood Separator). Remove plasma or serum promptly from the cells or clot.
If plasma is being collected, use only lithium-heparinized samples.
When testing CRP on patients with suspected severe systemic inammation, dilutions of the sample
may be performed to avoid repeat testing when CRP values are above 10.0 mg/dL (100.0 mg/L). The
recommended dilution is one part serum or plasma in one part normal saline (0.9% saline). IDEXX
recommends only diluting tests with results outside of the reportable range. Diluting tests with results in
the normal range may produce invalid results.
Note: Whole blood samples processed in the whole blood separator should not be diluted.
CRP cannot be run with the Phenobarbital (PHBR) test.
Complementary Tests
CRP should be evaluated in conjunction with a comprehensive history, physical examination, complete
blood count, complete biochemical prole, and urinalysis to provide a comprehensive database when
suspecting systemic inammation. If infection is suspected, detecting of the pathogen is needed to make a
nal diagnosis.
Fructosamine (FRU)
Fructosamine is glycated albumin or other proteins. Its concentration is related to blood glucose
concentration during the preceding 2 to 3 weeks.
Principal Reason for Performing the Test
Measurement of fructosamine concentration as part of the routine evaluation of a diabetic patient
undergoing therapy. It provides information about the status of glycemic control during the 2–3 weeks
prior to evaluation. In cats, fructosamine concentrations can be measured to identify if a stress response
or diabetes mellitus is the reason for high blood glucose concentrations. In addition, during management
of diabetes in both canine and feline patients, fructosamine concentration is used to clarify discrepancies
between the history and physical examination ndings and serial blood glucose concentration
measurements and it is also used to assess the effectiveness of therapy.
Appendices

41
Most Common Abnormality Indicated by the Test
Increased fructosamine indicates lack of or inadequate glucose regulation due to diabetes mellitus.
Fructosamine concentrations increase with poor glycemic control and decrease when glycemic control
improves. Less common, a low fructosamine may indicate prolonged hypoglycemia.
Sample Type and Precautions
Samples acceptable for FRU measurement include serum, plasma, and whole blood (when using the
Catalyst Lithium Heparin Whole Blood Separator). Remove plasma or serum promptly from the cells or
clot. If plasma is being collected, use only lithium heparinized samples. If you cannot perform FRU analysis
within 4 hours of sample collection, store the processed serum in the freezer (-18°C [0°F]) for up to 1 month.
It is important to separate the sample from the red blood cells as promptly as possible.
Serum is preferred for fructosamine testing as customer experience shows that it more consistently
provides good quality samples.
Examine the serum or plasma for hemolysis. Although IDEXX dry-slide technology dramatically reduces
the effect of this interfering substance, marked hemolysis can result in inaccurate fructosamine results.
Typically, marked hemolysis will lower the reported value on the Catalyst analyzers.
Reaction Sequence
Gamma-glutamyltransferase (GGT)
The enzyme gamma-glutamyltransferase is membrane-bound. It is present in large quantities in the kidney
medulla and cortex and to a lesser extent in the small intestinal mucosa and bile ductular epithelium.
Despite the high activity of gamma-glutamyltransferase in the kidney, renal disease does not result in high
enzyme activity in the serum sample. GGT in the kidney is primarily related to tubular lining epithelial cells
and the enzyme is localized to the apical portion of the cell. Pathologic changes in these tubular epithelial
cells result in loss of GGT directly into the urine. Measurement of GGT in the urine can prove to be a
sensitive indicator of tubular epithelial cell injury/nephrotoxicity.
Principal Reason for Performing the Test
As an indicator of cholestasis or gallbladder disease.
Most Common Abnormality Indicated by the Test
Increased GGT—cholestasis.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is being collected, use
only lithium heparinized samples. Hemolyzed specimens should not be used. Do not use
uoride/oxalate as an anticoagulant.
Complementary Tests
Serum gamma-glutamyltransferase activity is usually determined in conjunction with other tests of hepatic
function or damage.
Reaction Sequence
Appendices

42
Glucose (GLU)
Glucose is the principal source of energy in monogastric mammals. The circulating concentration in the
healthy animal is maintained within narrow limits.
Principal Reason for Performing the Test
To investigate carbohydrate metabolism.
Most Common Abnormality Indicated by the Test
Increased glucose—diabetes mellitus; glucocorticoid inuence; epinephrine inuence.
Sample Type and Precautions
For glucose determinations, the animal should have been fasted for 5–8 hours before sampling. Hemolysis
may affect glucose results.
For plasma samples: Use only lithium heparinized samples. When blood is collected in lithium heparin,
it is important that the sample be centrifuged immediately after collection. In this anticoagulant,
glycolysis occurs quite rapidly in the presence of red blood cells and the glucose concentration in the
sample can diminish at up to 10% an hour at room temperature. Remove plasma promptly from the red
blood cells. Hemolyzed specimens should not be used.
For serum samples: Do not centrifuge serum samples until clotting is complete. Samples must be
centrifuged completely. Remove serum promptly from the clot to avoid metabolism of glucose by the
cells. A maximum of 30 minutes between drawing and separation from the clot is recommended.
Hemolyzed specimens should not be used.
Complementary Tests
When the patient is a diagnosed diabetic, glucose tests may be performed in isolation. It is, however, useful
to perform other tests for renal and hepatic function and lipid metabolism to monitor secondary effects
of poorly controlled diabetes. Because stress in companion animals, particularly cats, can signicantly
raise glucose above the reference range, a fructosamine level should be considered in suspected cases of
diabetes mellitus. A concurrent urinalysis should also be performed to evaluate for the presence of glucose
and ketones.
Reaction Sequence
Inorganic Phosphate (PHOS)
Phosphorus plays a major role as a metabolic intermediate and is a constituent of nucleic acids,
phospholipids, and nucleotides. Phosphates are also important components of buffering systems within
the body uids. Phosphate and calcium are absorbed in the small intestine. Absorption is inuenced by the
presence of other minerals, nutrients, vitamins, and intestinal pH. Calcium and phosphorus metabolism are
interdependent.
Principal Reason for Performing the Test
As a measure of glomerular ltration rate.
Most Common Abnormality Indicated by the Test
Increased inorganic phosphate—decreased glomerular ltration.
Appendices

43
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is being collected, use only lithium
heparinized samples. Do not use oxalate, uoride, citrate, or EDTA as anticoagulants. Blood samples must
be processed and centrifuged as soon as possible after collection as phosphates are released quickly from
the red blood cells. Hemolysis can result in marked increases in phosphate concentration.
Complementary Tests
Inorganic phosphate determination should be performed in conjunction with measurements of calcium,
albumin, total protein, and glucose. If renal disease is suspected, BUN, creatinine, albumin, total protein, and
a complete urinalysis should also be determined.
Reaction Sequence
Lactate Dehydrogenase (LDH)
The enzyme lactate dehydrogenase is present in large amounts in all organs and tissues (including red
blood cells) of most animals. It is found in the cell cytoplasm and is released into the blood during reversible
and irreversible (necrosis) cell injury. The test is not a specic or sensitive indicator of damage to any organ
or tissue.
Note: The normal range of lactate dehydrogenase in the dog and cat is wide, as can be the intra-animal
variation from day to day. Consequently, small increases in activity due to minimal organ damage are
dicult to identify. The measurement of lactate dehydrogenase is a somewhat traditional test whose
diagnostic value is limited in practice.
Principal Reason for Performing the Test
To investigate damage to liver, cardiac or skeletal muscle.
Most Common Abnormality Indicated by the Test
Increased activity is usually associated with hepatic parenchymal lesions.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot and analyze as soon as possible. If plasma is
being collected, use only lithium heparinized samples. Fluoride/oxalate and EDTA should not be used as
anticoagulants.
Hemolyzed specimens should not be used because LDH contamination from red blood cells will occur.
Complementary Tests
Lactate dehydrogenase activity is usually determined in conjunction with other tests of liver, cardiac, or
skeletal muscle function or damage.
Reaction Sequence
Appendices

44
Lactate (LAC)
Lactate is produced by anaerobic metabolism of glucose and its concentration depends on relative rates of
production in muscle cells and erythrocytes and metabolism in the liver.
Principal Reason for Performing the Test
Increased lactate levels usually are caused by overproduction or under metabolism. They result from tissue
hypoxia, diabetes mellitus, malignancies, ethanol or methanol ingestion, and metabolic acidosis.
Most Common Abnormality Indicated by the Test
Hypoxia secondary to severe exercise, shock, hypovolemia, cardiac disease, pulmonary edema, and
seizures.
Sample Type and Precautions
Use lithium heparinized or Fl/oxalated samples. When using lithium heparinized samples, separate the
plasma from the red cells within 5 minutes of collection.
Complementary Tests
CBC, biochemical panel, complete urinalysis, and blood gas.
Reaction Sequence
Lipase (LIPA)
Lipase is secreted by the pancreas and to a lesser extent by the gastrointestinal mucosa. Lipase is a
relatively sensitive indicator of pancreatic pathology (as compared to amylase). Generally a greater than
threefold increase above the reference range is supportive of pancreatitis.
Principal Reason for Performing the Test
As an indicator of acute pancreatitis.
Most Common Abnormality Indicated by the Test
Acute pancreatitis.
Sample Type and Precautions
Blood samples should be taken within one day of the onset of symptoms suggesting acute pancreatitis.
Promptly remove plasma or serum from the cells or clot. If plasma is being collected, use only lithium
heparinized samples. Do not use oxalate/uoride, citrate, or EDTA anticoagulants. Lipemia and icterus may
increase lipase results.
Complementary Tests
Lipase and amylase are usually determined in conjunction with tests of hepatic and pancreatic function or
damage. Canine and feline pancreas-specic lipase tests should be performed in questionable cases.
Appendices

45
Reaction Sequence
Magnesium (Mg)
Magnesium plays an important intracellular role in the activation of enzymes including those responsible
for many anabolic and catabolic processes. It is also involved in the formation and destruction of
acetylcholine, which governs the transmission of electrical impulses at the neuromuscular junction. The
adrenal, thyroid, and parathyroid glands appear to regulate serum magnesium concentration.
Principal Reason for Performing the Test
The importance of measuring serum magnesium concentration in dogs and cats has not been fully
investigated. However, there have been reports of hypomagnesemia in dogs following the removal of the
parathyroid gland.
Most Common Abnormalities Indicated by the Test
Increased magnesium—decreased glomerular ltration.
Decreased magnesium —parathyroid gland removal.
Sample Type and Precautions
Blood samples should be centrifuged immediately after collection as magnesium is released from
hemolyzed erythrocytes and can give erroneously high magnesium results. Remove plasma or serum
promptly from the cells or clot. If plasma is being collected, use only lithium heparinized samples. Do not
use oxalate/citrate or EDTA as anticoagulants. Blood collection tubes preserved with sodium uoride cause
lower results.
Reaction Sequence
Appendices

46
Pancreatic Lipase (PL)
Pancreatic lipase is a digestive enzyme produced by the pancreas to hydrolyze lipids. Under normal
circumstances, only small amounts of pancreatic lipase are found in the circulation. When the pancreas
undergoes inammation or damage (i.e., neoplasia, trauma), an increased amount of pancreatic lipase is
released and is an indicator of pancreatic pathology.
Principal Reason for Performing the Test
To diagnose and monitor pancreatitis in sick patients.
Most Common Abnormalities Indicated by the Test
Acute or chronic pancreatitis.
Sample Types and Precautions
+ Catalyst* Pancreatic Lipase supports the use of serum, lithium heparin plasma, and whole blood (using
the Catalyst Lithium Whole Blood Separator). Remove plasma or serum promptly from the cells or clot.
+ Catalyst Pancreatic Lipase is robust to lipemia and icterus.
+ Moderate-to-marked hemolysis can result in decreased Catalyst Pancreatic Lipase results.
+ Be careful not to aspirate cells during serum/plasma preparation, and ensure the Catalyst Lithium Whole
Blood Separator is lled with 0.7 cc to prevent overlling.
Complementary Tests
Catalyst Pancreatic Lipase should be evaluated in conjunction with a comprehensive history, physical exam,
complete blood count, complete biochemical prole, and urinalysis to evaluate for evidence of systemic
complications of pancreatitis and comorbidities.
Reaction Sequence
Phenobarbital (PHBR)
Phenobarbital is a commonly used drug used to treat seizures in a variety of species. Phenobarbital levels
should be evaluated during initial dosing and throughout treatment to ensure that the blood levels are within
the targeted therapeutic range.
Principal Reasons for Performing the Test
Phenobarbital is a controlled barbiturate medication that is used to treat veterinary patients that have
seizures. The dosage of phenobarbital needs to remain within a specic range to be effective. If the level is
<10 µg/mL, there may not be a sucient level of phenobarbital to prevent seizures. If the level >30 µg/mL in
cats or >40 µg/mL in dogs, phenobarbital can be toxic and potentially life threatening.
In most patients, steady state is achieved after 2–3 weeks of consistent dosing with phenobarbital. Once
steady state is achieved, timing of sample collection is not important in more than 90% of patients.
However, there can be variability of the phenobarbital half-life in a small percentage of patients. Therefore,
if toxicity is suspected, a peak sample (4–5 hours post-pill) may be helpful, and if breakthrough seizures are
occurring and inadequate dosing is suspected, a trough level (collected immediately prior to the next dose)
may be helpful.
Appendices

47
Therapeutic monitoring should be performed after two to four weeks of consistent dosage following
initiation of treatment or dosage change to allow most patients to achieve a relatively steady state. Patients
on lower doses (mg/kg) may take longer to come to steady state. Consistent timing of sampling remains
important for comparison across time as there may still remain some uctuation throughout the day,
especially for patients receiving higher doses. Monitoring should then be repeated at a minimum of every
six months thereafter, depending on clinical response.
Most Common Abnormalities Indicated by the Test
Over or under dosage of medication.
Sample Type and Precautions
Do not use separator tubes as contact with the gel may decrease levels.
Complementary Tests
CBC, full chemistry panel, urinalysis, bile acids (minimally 2 times per year)
Reaction Sequence
Potassium (K)
Potassium is the major cation of intracellular uid, where it is the major buffer within the cell, facilitates
nerve conduction and muscle function, and helps maintain osmotic pressure. Abnormally high or low
potassium levels cause changes in muscle irritability, respiration, and myocardial function.
Principal Reasons for Performing the Test
High potassium (hyperkalemia) is usually found in urinary obstruction, renal failure, metabolic or respiratory
acidosis, and hypoadrenocorticism as well as excessive hemolysis for horses, cattle, cats, and some
breeds of dogs. Decreased values (hypokalemia) usually follow excessive salt loss through severe vomiting
or diarrhea, inadequate intake, anorexia (especially cats), malabsorption, and severe burns.
Most Common Abnormalities Indicated by the Test
Hyperkalemia—renal failure, postrenal obstruction.
Hypokalemia—excessive loss of potassium.
Sample Type and Precautions
Remove plasma or serum promptly from cells or clot. If plasma is being collected, use only lithium
heparinized samples. Avoid hemolysis. Potassium bromide may increase Catalyst electrolyte results.
Do not freeze samples for use with the Catalyst One analyzer.
Complementary Tests
Sodium, potassium, and chloride should always be assayed together to determine electrolyte balance. The
additional measurement of bicarbonate will allow accurate assessment of metabolic acid-base physiology.
ACTH stimulation test for suspect cases of hypoadrenocorticism.
Appendices

48
Reaction Sequence
Progesterone
Progesterone is a female reproductive hormone. In the bitch, increased production occurs during late
proestrus, through estrus, and into diestrus. It is necessary for the maintenance of pregnancy in most
species.
Principal Reason for Performing the Test
In the bitch, uses of progesterone testing include:
+ Predicting (and later conrming) ovulation for timing of breeding.
+ Predicting parturition date and/or time of cesarean section.
+ Investigating reproductive abnormalities.
Sample Type and Precautions
Catalyst Progesterone has been optimized for use with canine whole blood (using the Catalyst* Lithium
Heparin Whole Blood Separator) and lithium heparin plasma samples. Serum is also acceptable. It is
important to remove plasma or serum promptly (within 30 minutes) from the red blood cells or clot.
+ If plasma is being collected, use only lithium heparinized samples.
+ If serum is being collected, do not use a serum separator tube (SST) as the gel interferes with
progesterone testing.
+ Catalyst Progesterone is robust to icterus and lipemia. Marked hemolysis (obvious on visual inspection
of the serum/plasma) can result in inaccurate progesterone results (falsely low).
+ The sample should not be diluted.
+ Serial progesterone concentrations should be monitored using a consistent sample type and handling
method.
+ Catalyst Progesterone was designed to measure naturally occurring progesterone in canine samples.
Use of progesterone supplementation may impact results.
Do not expose progesterone tests to topical progesterone products (e.g., creams applied to human skin). If
these creams have been used, the operator should wear clean, powder-free latex or nitrile gloves whenever
using the Catalyst Progesterone Test or the Catalyst One* or Catalyst Dx* analyzers. Tests exposed to
progesterone products may experience an increased reported value on the Catalyst One and Catalyst Dx
analyzers.
Complementary Tests
To increase the accuracy of predicting ovulation and timing breeding:
+ Trend progesterone results over many days taking care to be consistent with sample type and handling.
+ Use progesterone trends in combination with vaginal exfoliative cytology.
+ Monitor (once or twice daily) for the onset of vulvar softening.
To increase the accuracy of determining parturition date:
+ Trend progesterone results over many days taking care to be consistent with sample type and handling.
+ Use progesterone trends in combination with knowledge of mating events, repeated measurement of
body temperature, and observation of clinical signs.
+ Before cesarean section, conrm a persistent decrease in progesterone concentrations with repeat
testing.
Appendices
49
For some cases, the addition of LH (luteinizing hormone) testing may be useful, particularly when using
frozen semen for articial insemination.
Different methods for measuring progesterone have differing performance and it is important to use the
interpretive comments supplied with the relevant test. When trending progesterone results to determine
ovulation timing, always use one methodology and sample type. Decisions regarding breeding should not
be made based on progesterone testing alone.
Sodium (Na)
Sodium is the major cation of extracellular uid, where it maintains osmotic pressure, acid-base balance,
and transmits nerve impulses. The body maintains total sodium content, and only slight changes are found
even under pathologic conditions.
Principal Reasons for Performing the Test
To evaluate electrolyte status in conjunction with potassium and chloride levels.
Low sodium (hyponatremia) is usually caused by a relative excess of body water. Reduced levels may be
due to low intake, loss through vomiting or diarrhea plus adequate water and inadequate salt replacement,
salt-losing nephropathy, osmotic diuresis, metabolic acidosis, and various glandular conditions.
Increased values (hypernatremia) usually follow water loss in excess of salt loss through profuse sweating,
severe vomiting or diarrhea, inadequate water intake, and dehydration of renal sodium conservation in
hyperaldosteronism.
Most Common Abnormality Indicated by the Test
Hypernatremia secondary to dehydration, gastrointestinal uid loss (vomiting or diarrhea).
Sample Type and Precautions
Remove plasma or serum promptly from cells or clot. If plasma is collected, use only lithium heparinized
samples. Avoid hemolysis. Potassium bromide may increase Catalyst electrolyte results.
Do not freeze samples for use with the Catalyst One analyzer.
Complementary Tests
Sodium, potassium, and chloride should always be assayed together to determine electrolyte balance. The
additional measurement of bicarbonate will allow accurate assessment of metabolic acid-base physiology.
Reaction Sequence
Symmetric dimethylarginine (SDMA)
Symmetric dimethylarginine (SDMA) is a stable molecule that originates from posttranslational methylation
of arginine residues of intranuclear cellular proteins integral to basic cellular metabolism, and subsequent
protein degradation. SDMA production is constant and is largely unaffected by body condition, advanced
age, diet, exercise, disease state, or catabolism. SDMA is eliminated from the body by glomerular ltration
in the kidneys.
Principal Reason for Performing the Test
SDMA is a sensitive biomarker of glomerular ltration rate. SDMA increases earlier than creatinine as
kidney function declines and, unlike creatinine, SDMA is not impacted by non-renal factors, such as lean
muscle mass or diet.
Appendices
50
Most Common Abnormality Indicated by the Test
Increased SDMA indicates reduced glomerular ltration rate due to prerenal (dehydration, hypotension),
renal (acute and active kidney injury and/or chronic kidney disease), or postrenal (urinary obstruction)
conditions.
Sample Type and Precautions
Samples acceptable for the Catalyst* SDMA Test include canine and feline serum, plasma, and whole blood
(when using the Catalyst Lithium Heparin Whole Blood Separator). Remove plasma or serum promptly from
the cells or clot. If plasma is being collected, use only lithium heparinized samples. The sample should not
be diluted.
Complementary Tests
Changes in kidney function associated with increased SDMA should be acted on immediately and
evaluated considering the clinical presentation and physical examination ndings. Complementary
laboratory testing begins with a complete urinalysis and complete biochemical prole, including creatinine,
BUN, inorganic phosphate, total protein, albumin, and electrolytes. A complete blood count is suggested.
Probable kidney disease should be investigated for an underlying cause with a urine culture and MIC
susceptibility, infectious disease testing, and diagnostic imaging, as well as a search for exposure to kidney
toxins or nephrotoxic medications. Patients with increased SDMA should also be assessed for confounding
conditions by measuring blood pressure and a urine protein to creatinine ratio and by testing thyroid
function.
Total Bilirubin (TBIL)
Hemoglobin from degenerated erythrocytes is converted to bilirubin in the monocyte-macrophage
system. Free unconjugated bilirubin is transported to the liver bound to albumin, where it is conjugated
with glucuronic acid and eliminated in the bile. In obstructive liver disease, the concentration of conjugated
bilirubin in the blood increases.
During intravascular or extravascular hemolysis, very large numbers of erythrocytes may be destroyed
quickly and the conjugation mechanism in the liver may become overloaded so that high concentrations
of unconjugated bilirubin are found in the blood. If the loss of hemoglobin and erythrocytes is very large,
anoxia may occur. Hepatocyte dysfunction follows leading to cellular swelling, which occludes the bile
canaliculi preventing the elimination of conjugated bilirubin. A concomitant rise in circulating conjugated
bilirubin then occurs.
Principal Reason for Performing the Test
To detect hepatobiliary disease and excessive erythrocyte destruction.
Note: In healthy dogs and cats, the concentration of total bilirubin in the serum is very low. Visual inspection
of the sample will frequently indicate whether bilirubin determination is necessary (serum and plasma only).
Most Common Abnormality Indicated by the Test
Increased bilirubin—cholestatic liver disease (conjugated bilirubin) and hepatic insuciency (unconjugated
bilirubin), hemolytic disease (unconjugated and possible conjugated bilirubin), and intrahepatic obstruction.
Sample Type and Precautions
Remove plasma or serum promptly from cells or clot. Samples should be analyzed immediately as bilirubin
degrades rapidly in light. If immediate analysis is impossible, the sample must be kept in the dark and
preferably at 4°C–8°C (36°F–40°F) in a refrigerator. Sample must be allowed to come to room temperature
before analysis. If plasma is collected, use only lithium heparinized samples.
It is critical that samples be properly centrifuged. Otherwise, leukocytes and platelets may remain in
suspension, even when red blood cells have been separated. Cellular material on the slide may cause
signicant positive error. Also, hemoglobin increases total bilirubin results, so avoid even moderately
hemolyzed samples.
Appendices

51
Complementary Tests
Total bilirubin should be determined with other tests of hepatic function or damage. Hematocrit should
also be performed to eliminate or conrm the presence of hemolytic disease. Determination of urinary
urobilinogen and bilirubin may also be useful.
Reaction Sequence
Total Protein (TP)
The serum total protein concentration comprises all the proteins found in the aqueous phase of the blood.
In healthy animals, albumin is the major single component. The remaining proteins are the alpha, beta,
and gamma globulins. The globulin concentration is determined by subtracting the albumin from the total
protein.
Principal Reason for Performing the Test
Total protein measurement may provide useful information when used in combination with tests
to investigate hepatic and renal function, the degree of hydration, protein-losing enteropathies, or
gammopathies. The test is nonspecic and, if performed in isolation, will be unlikely to provide diagnostic
information.
Most Common Abnormalities Indicated by the Test
Increased total protein—dehydration, inammatory disease.
Decreased total protein—loss of proteins through blood loss and gastrointestinal loss, decreased albumin
associated with protein-losing nephropathy and enteropathy, and decreased albumin associated with
hepatic insuciency and inammatory disease.
Impaired renal and hepatic function, dehydration, and gastrointestinal lesions.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is collected, use only lithium
heparinized samples. Moderate-to-marked hemolysis can result in false high total protein concentration.
Results obtained from the analysis of plasma may be slightly higher than serum due to the brinogen that
remains in the plasma.
Complementary Tests
Total protein concentration is usually determined in conjunction with the measurement of albumin and
other tests of renal and hepatic function.
Reaction Sequence
Total T
4
(TT
4
)
An enzyme-linked immunosorbent assay (ELISA) for the quantitative measurement of total T
4
(thyroxine) in
canine and feline patients. With a total T
4
test, you can assess thyroid function, provide comprehensive one-
visit screening for feline hyperthyroidism, presumptive canine hypothyroidism, as well as monitor response
to treatment and adjust dosages immediately.
Appendices
52
Principal Reason for Performing the Test
To screen, diagnose, and monitor thyroid disease. The measurement of total thyroxine helps veterinary
practitioners to assess thyroidal function by measuring the bound and unbound thyroxine in the blood.
Thyroxine is the principal hormone secreted by the thyroid gland and is critical to metabolic processes.
Most Common Abnormality Indicated by the Test
Hyperthyroidism—an increased TT
4
is consistent with hyperthyroidism. Naturally occurring
hyperthyroidism is a common endocrine disorder in cats and rare in dogs.
Hypothyroidism—a decreased TT
4
is consistent with but not necessarily denitively diagnostic of
hypothyroidism. Naturally occurring hypothyroidism is a common endocrine disorder in dogs and rare in
cats.
Nonthyroidal illness (NTI)—nonthyroidal illness can affect TT
4
levels (and potentially other thyroid tests as
well). Nonthyroidal illness can lower TT
4
levels, potentially into the hypothyroid range. The more severe the
nonthyroidal illness, the greater the potential impact on TT
4
levels.
Sample Type and Precautions
For use with serum, plasma, and whole blood (when using the Catalyst Whole Blood Separator).
Remove plasma or serum promptly from the cells or clot. If plasma is being collected, use only lithium
heparinized samples. Do not use uoride/oxalate as an anticoagulant.
Complementary Tests
Total T
4
should be evaluated in conjunction with a comprehensive history, physical examination, CBC,
complete biochemical prole, and urinalysis to provide a comprehensive database of information in the
diagnosis or suspicion of thyroid disease.
In dogs with low or low normal T
4
results and with consistent clinical signs, evaluate free T
4
(fT
4
) and
endogenous thyroid-stimulating hormone (TSH) and possibly thyroglobulin autoantibodies (TgAA) to aid in
conrming hypothyroidism.
Cats with consistent clinical signs and total T
4
(TT
4
) values in the borderline high range (gray zone) may
have early hyperthyroidism or a concurrent nonthyroidal illness (NTI). In these cases, consider a free T
4
(fT
4
), a T
3
suppression test or radionuclide thyroid imaging to aid in conrming the diagnosis.
Triglycerides (TRIG)
Triglycerides are usually present in the diet of dogs and cats, especially when the animals are fed table
scraps. They are also synthesized in the liver, mainly from carbohydrates, to provide a secondary energy
source and are stored in fatty tissue. Their hydrolysis to mono- and diglyceride glycerol and free fatty acids
is catalyzed by pancreatic lipase.
Principal Reason for Performing the Test
To detect abnormalities in lipid metabolism.
Most Common Abnormality Indicated by the Test
Increased triglycerides—High-fat diet or abnormalities in fat metabolism.
Sample Type and Precautions
Blood should not be drawn within 12 hours of a meal.
Remove plasma or serum promptly from the cells or clot. If plasma is collected, use only lithium
heparinized samples. Grossly lipemic specimens probably have very high triglycerides and should be
diluted before analysis.
Appendices

53
Complementary Tests
Triglycerides should not be measured in isolation. If the sample is turbid or milky, the test should be
determined in conjunction with measurements of cholesterol and glucose, and hepatic and renal function
tests. Also consider repeat sampling if the patient has not been fasted for 12 hours.
Reaction Sequence
Uric Acid (URIC)
Uric acid determinations are useful in avian patients and dalmatians in place of urea determinations. In all
dogs (except dalmatians) with diffuse hepatic disease, there is marked elevation of blood uric acid above
the normal levels of <1 mg/dL.
Principal Reason for Performing the Test
As an indicator of the severity of renal disease in avian populations (and dalmatians).
Most Common Abnormality Indicated by the Test
Increased uric acid—prerenal, postrenal, and renal azotemia associated with decreased glomerular ltration
rate.
Sample Type and Precautions
Remove plasma or serum promptly from the cells or clot. If plasma is collected, use only lithium
heparinized samples. Plasma collected from sodium uoride, citrate, or EDTA preservative should not be
used.
Complementary Tests
Creatinine, UCRE/CREA, UPRO
Reaction Sequence
Appendices

54
Urine Creatinine (UCRE)
Urine creatinine is determined so that the concentration of electrolytes ltered or lost through the glomeruli
or renal tubules, such as urinary protein or cortisol, can be quantitated, compared, and expressed as ratios
with diagnostic signicance.
Principal Reason for Performing the Test
To be performed with urine protein in order to determine the urine protein:creatinine ratio (UPC).
Most Common Abnormality Indicated by the Test
Proteinuria indicating early renal disease, protein-losing nephropathy.
Sample Type and Precautions
Centrifuged urine, preferably collected through cycstocentesis, collected in a clean container. An inactive
urinary sediment should be demonstrated and urinary tract infection (UTI) via culture and sensitivity should
be ruled out before performing, as UTI may mildly to moderately raise the UPC.
Complementary Tests
Complete urinalysis with culture and sensitivity. Serum chemistries, such as creatinine, BUN, albumin, and
globulin.
CBC
SNAP* 4Dx* Plus Test
Storage Information
Urine samples should be run within 2 hours of collection and can be stored in a refrigerator for up to 24
hours. DO NOT freeze urine samples.
Reaction Sequence
Urine Protein (UPRO)
Urinary protein is determined and compared to the concentration of creatinine in order to assess the level
of renal protein (glomeruli and tubular) loss to determine the urine protein:creatinine (UPC) ratio.
Principal Reason for Performing the Test
To be performed with urine creatinine in order to determine the urine protein:creatinine (UPC) ratio.
Most Common Abnormality Indicated by the Test
Proteinuria indicating early renal failure, protein-losing nephropathy.
Appendices

55
Sample Type and Precautions
Centrifuged urine, preferably collected through cycstocentesis, collected in a clean container. An inactive
urinary sediment should be demonstrated and urinary tract infection (UTI) via culture and sensitivity should
be ruled out before performing as UTI may mildly to moderately raise the UPC.
Complementary Tests
Complete urinalysis with culture and sensitivity. Serum chemistries such as creatinine, BUN, albumin, and
globulin.
CBC
SNAP* 4Dx* Plus Test
Storage Information
Urine samples should be run within 2 hours of collection and can be stored in a refrigerator for up to 24
hours. DO NOT freeze urine samples.
Reaction Sequence
Medical Protocol Descriptions
Ammonia Protocol
Baseline ammonia levels should be assessed in animals with signs of hepatic encephalopathy or in patients
suspected of having portosystemic shunts (PSS). Ammonia tolerance tests may be considered to evaluate
for PSS where bile acids are not considered (for example, in Maltese).
Ammonia tolerance test: A baseline sample is drawn after the patient has been fasted for
12 hours. Ammonium chloride (0.1 g/kg) by mouth via stomach tube or gelatin capsules.
A second sample is drawn 30 minutes after ammonium chloride administration.
Note: Vomiting during the procedure will invalidate results.
Sample Requirements: 1 mL heparinized plasma, separated from RBCs. Do not use serum.
Storage/Stability: Samples must be analyzed immediately after collection. If there is any delay between
collection, centrifugation, and analysis, the sample must be capped and placed on ice immediately.
Interferences: Hemolysis, glucose levels over 600 mg/dL (33.33 mmol/L), high BUN values
Comments: Anticoagulated blood must be centrifuged immediately after collection. Separate plasma and
place it in a glass container (RTT). Freeze immediately and keep frozen if not running sample immediately.
Note: Ammonia levels increase with time.
UPC Protocol
Principal Reason for Performing Test: To aid in the diagnosis of protein-losing nephropathies such as
glomerulonephritis and amyloidosis and as an early marker of chronic renal failure.
Includes: Urine protein (UPRO), urine creatinine (UCRE), protein:creatinine (UPC) ratio
Sample Requirements: 2 mL urine in a sterile container
Storage/Stability: 48 hours at 2°C–8°C (36°F–46°F)
Interferences: Gross hematuria, pyuria.
Complementary Tests: Complete urinalysis with culture and sensitivity. Serum chemistries such as
creatinine, BUN, albumin, globulin; CBC; SNAP* 4Dx* Plus Test; and imaging studies.
Appendices
56
Interpretation: Proteinuria requires proof of persistence and localization to prerenal, renal, or postrenal origins.
Prove persistence of proteinuria by repeating the UPC ratio at least three times, a minimum of 2 weeks apart.
+ Prerenal proteinuria is possible when a CBC and a biochemical prole detect hemolysis,
hyperglobulinemia or evidence of muscle damage. Recommend investigation and management for the
underlying cause.
+ Postrenal proteinuria is caused by urogenital tract diseases, hematuria, or pyuria. Repeat the test with
a cystocentesis sample or evaluate urine sediment for hemorrhage or inammation. Consider a urine
culture. Recommend investigation and management for the underlying cause.
+ Renal proteinuria: evaluate in the face of azotemia.
Nonazotemic, persistent, renal proteinuria (dogs and cats):
UPC <0.5 = within reference range
UPC 0.5–1.0 = questionable, repeat at appropriate range
UPC 1.0–2.0 = excessive proteinuria; recommend investigation for underlying systemic diseases
UPC 2.0 = excessive proteinuria; recommend investigation for underlying systemic diseases and medical
management
Azotemic, persistent, renal proteinuria (dogs):
UPC <0.5 = warrant monitoring and investigation
UPC ≥0.5 = excessive proteinuria; recommend investigation for underlying systemic diseases and medical
management
Azotemic, persistent, renal proteinuria (cats):
UPC <0.4 = warrant monitoring and investigation
UPC ≥0.4 = excessive proteinuria; recommend investigation for underlying systemic diseases and medical
management

57
Appendices

58
Appendices
Total T
4
Protocols
CBC = Complete blood count
Note: 1 µg/dL is equal to 12.87 nmol/L. A result
that falls within the low normal range of the
assay should be considered ambiguous.
Canine hypothyroidism suspected
Initial database
• Total T
4
• CBC
• Chemistry with
electrolytes
• Complete urinalysis
Low T
4
<1.0 µg/dL
(<13.0 nmol/L)
Low T
4
with NTI
<1.0 µg/dL
(<13.0 nmol/L)
Normal T
4
2.0–4.0 µg/dL
(26.0–51.0 nmol/L)
Hypothyroidism unlikely
fT
4
+
TSH
±
TgAA
Low fT
4
±
high TSH
±
positive TgAA
Hypothyroidism likely
Clinical trial
Hypothyroidism unlikely
Repeat testing in 4–6 weeks if
hypothyroidism still suspected
Normal fT
4
and TSH
negative TgAA
Address NTI
Low Normal T
4
1.0–2.0 µg/dL
(13.0–26.0 nmol/L)
Common clinical
signs in dogs
• Obesity
• Skin disease
• Lethargy
• Mental dullness
• Exercise/Cold
intolerance

59
Appendices
†
If strong suspicion of hyperthyroidism still exists,
consider retesting in 4–6 weeks or a technetium scan.
CBC = Complete blood count
Note: 1 µg/dL is equal to 12.87 nmol/L. A result that
falls within the gray zone of the assay should be
considered ambiguous.
Feline hyperthyroidism suspected
Normal T
4
0.8–4.7 µg/dL
(10.0–30.0 nmol/L)
Low T
4
<0.8 µg/dL
(<10.0 nmol/L)
High T
4
>4.7 µg/dL
(>60.0 nmol/L)
Hyperthyroidism
likely
Hyperthyroidism unlikely
†
Euthyroid sick
or iatrogenic
Low or
normal fT
4
High fT
4
Normal T
4
(gray zone)
2.3–4.7 µg/dL
(30.0–60.0 nmol/L)
Common clinical
signs in cats
• Weight loss
• Hyperactivity
• Polyphagia
• Palpable goiter
• Unkempt coat
Initial database
• Total T
4
• CBC
• Chemistry with
electrolytes
• Complete urinalysis
60
Differences in Results
With a Commercial Laboratory or Other Instrument
Reference ranges must be created for each analyte and each new instrument or method of analysis.
Every commercial laboratory must establish its own species reference ranges for the equipment and
methodology used. IDEXX is continually doing this work for you with every software release.
Comparing results from different laboratories that may be using different equipment or methods is
imprecise at best. Any comparisons should be performed on the same sample that has been “split,” stored
under like conditions, and tested at approximately the same time. Compare each result to the reference
range stated by IDEXX or the commercial laboratory (as appropriate). Each result should have the same
relationship to its method’s reference range. For instance, a sample giving a Catalyst One* result that is
slightly below the Catalyst One analyzer’s normal range should give a laboratory result slightly below the
laboratory’s normal range.
Technical Specications
Dimensions
Width: 10.0 inches
Depth: 14.8 inches
Height: 14.0 inches
Weight: approximately 25 pounds
Power Supply
Input: 100–240 V AC, 50–60 Hz, 2 Amps
Power Supply Protection: IPX0
Rated: 24VDC, 6.25A
Input/Output Connections
There are two user-accessible Input/Output connections on the rear of the Catalyst One analyzer (power
connection and Ethernet port for connection to IDEXX VetLab* Station).
Operating Conditions
Indoor use only
Altitude: Up to 2,000 meters
Operating Storage
Temperature 15°C–30°C (59°F–86°F) 5°C–38°C (41°F–100°F)
Relative Humidity 15%–75% 20%–85%
Appendices

61
IDEXX Customer and Technical Support contact information
United States/Canada 1-800-248-2483
Europe idexx.eu
Australia 1300 44 33 99
New Zealand 0800 83 85 22
Brazil 0800 -777-7027
Latin America sopor[email protected]
China (PRC) 400-678-6682
South Korea 080 7979 133
Taiwan 0800 291 018
Japan 0120-71-4921
